FLOATS OR SINKS DENSITY Density It is a


















- Slides: 25

FLOATS OR SINKS DENSITY

Density It is a measure of compactness of how much mass is tightly squeezed into a given volume. Is the ratio of mass and volume in an object. D= M/V g/ml or g/cm 3 2

DENSITY D = m/v (g/cm 3) (g/ml) Mass usually expressed in grams Volume usually expressed in cm 3 or milliliters, etc.

Density = amount of matter per unit volume § Density is the ratio of mass to volume § If the volume stays the same and the mass § increases. . . the density will increase § If the mass stays the same and the volume increases. . . The density will decrease

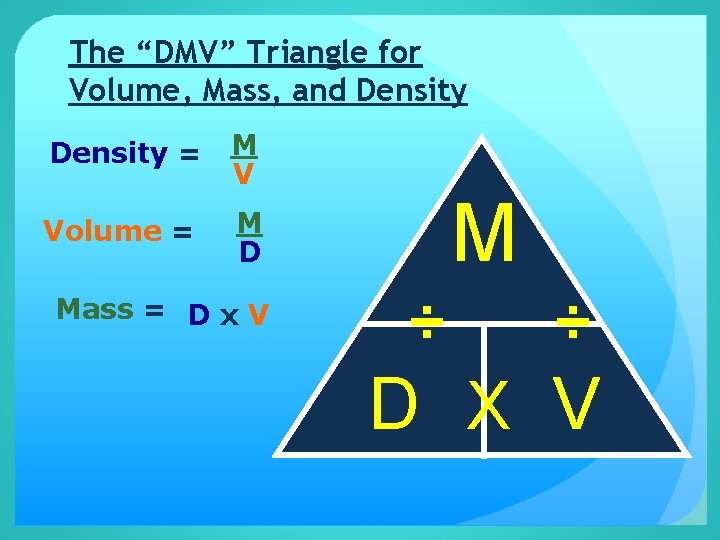
The “DMV” Triangle for Volume, Mass, and Density = M V Volume = M D Mass = D x V M ÷ ÷ D X V

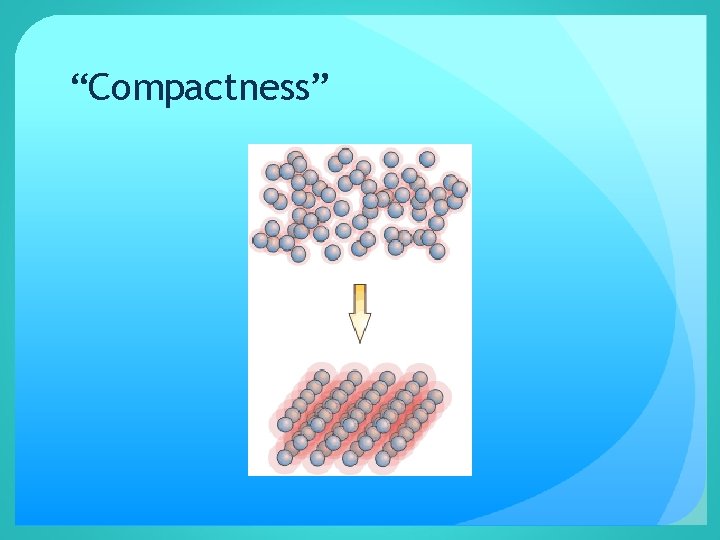
Density is the measure of the “compactness” of a material n. How close the atoms or molecules are to each other n. More than “heaviness” - density includes how much space an object takes up!! n. All substances have density including liquids, solids, and gases

What would take up more space? ? ? kilogram of feathers…. . or a kilogram of steel? ? OR A

“Compactness”

Density The density of water is 1. 0 g/ml Objects with densities greater than 1. 0 g/ml will sink in water 9

Density Objects with densities less than 1. 0 g/ml will float on water 10

Ice floats therefore it is less dense than water Ice mostly remains underwater with only a portion of it being exposed 11

Solids Ice vs. water…. .

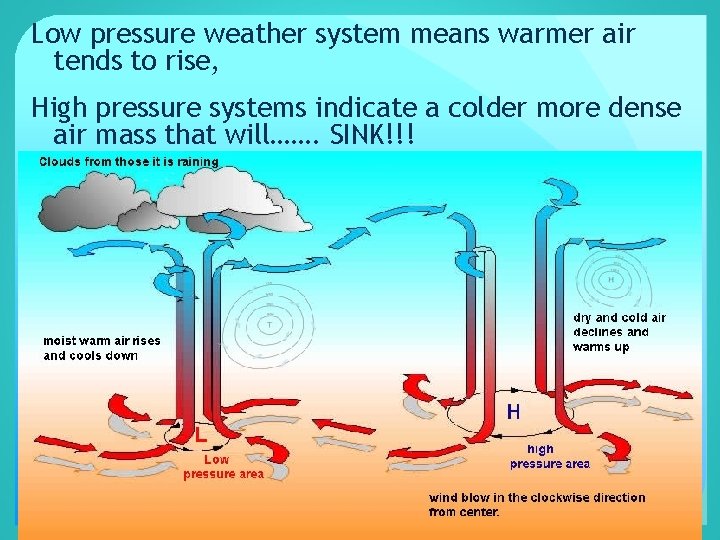
Gases n. How much kinetic energy do the molecules have? ? n. The greater the kinetic energy n ……the greater the volume n …… and the less dense that gas is!! n. Therefore, cold air is more dense than warm air n

Low pressure weather system means warmer air tends to rise, High pressure systems indicate a colder more dense air mass that will……. SINK!!!

LIQUIDS n The more dissolved solids in a solution, the more dense (such as ocean water) n Cold water in lakes tend to sink (this creates a constant mixing of water, nutrients, and other substances) Kinetic energy again!! Denser layers to less dense layers…. .

What would happen? ? n Mercury density = 13600 kg/m 3 n Lead density = 11340 kg/m 3

Lead floats on liquid mercury!

Solids Ice vs. water…. .

SOLIDS n Ice is less dense than water (which is why lakes and ponds have a thin layer of ice covering in winter, with water underneath) n Various rocks, woods, metals have a characteristic density specific to that substance Wouldn’t you like to have a bunch of THIS dense material?

Archimedes and the Kings Crown http: //3 quarksdaily. blogs. com/3 quarks daily/images/2007/07/18/archimede s. jpg

Calculations If 96. 5 grams of gold has a volume of 5 cubic centimeters, what is the density of gold? 21

Calculation If 96. 5 g of aluminum has a volume of 35 3 cm , what is the density of aluminum? 22

Calculation If the density of a diamond is 3. 5 g/cm 3, what would be the mass of a diamond whose volume is 0. 5 3 cm ? 23

What is specific gravity? A comparison of the density of a substance and the density of water is specific gravity 24

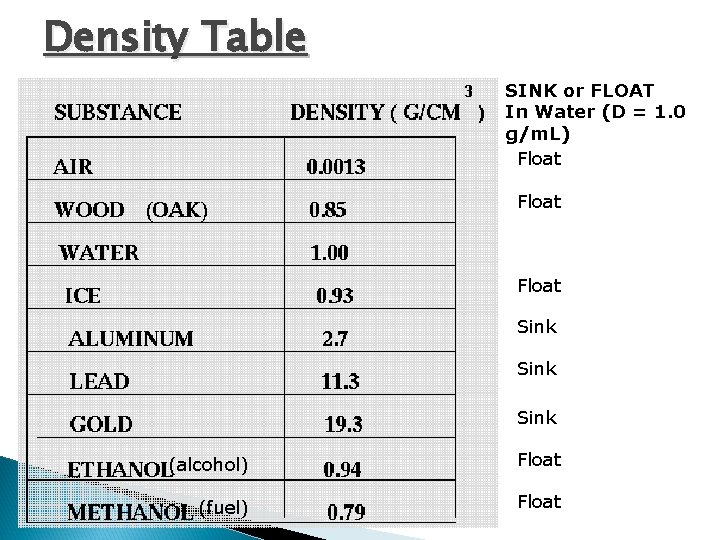
Density Table SINK or FLOAT In Water (D = 1. 0 g/m. L) Float Sink (alcohol) Float (fuel) Float