Flashcards for Unit 1 Matter Anything that has






































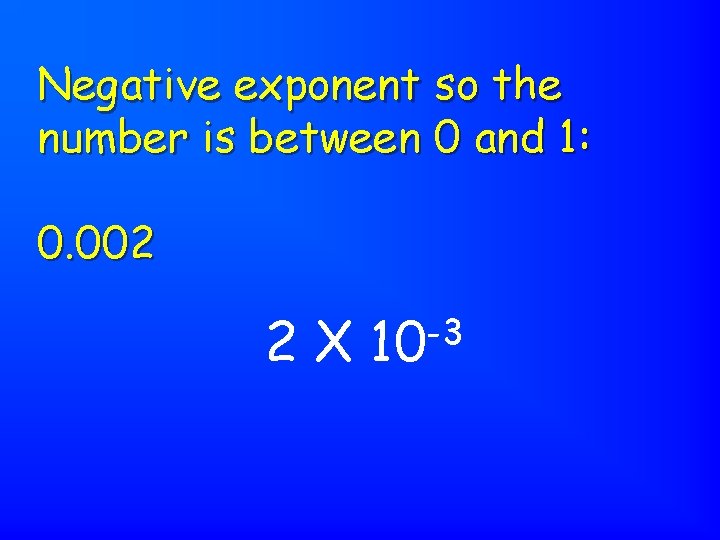













- Slides: 64

Flashcards for Unit 1

Matter Anything that has mass & occupies space.

A measure of the gravitational pull on matter. You would weigh less on the moon! Weight

A measure of the quantity of matter. You would have the same mass on the moon. Mass

A series of steps followed to solve problems, including collecting data, forming a hypothesis, testing the hypothesis, and stating conclusions. Scientific Method

A fact you take in with your senses. Observation

A testable statement Hypothesis

An inference Conclusion

Describes what happens. Often stated mathematically. Summarizes many (thousands of) observations. Scientific Law

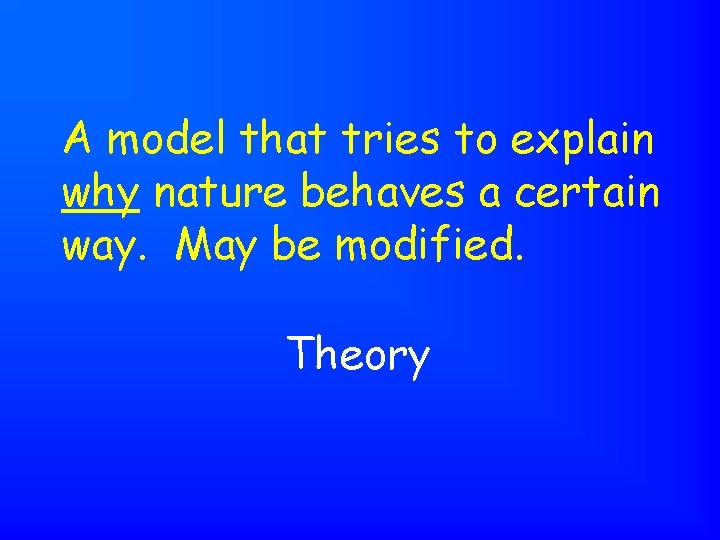
A model that tries to explain why nature behaves a certain way. May be modified. Theory

7 How many fundamental units in the SI system?

Fundamental or Base Unit Physical quantity that must be measured. It cannot be calculated.

Derived Unit A unit defined as a combination of fundamental units.

F i u t n s d a m e n t a l U n Unit of Mass Kilogram Unit of Time Second Unit of Length Meter Unit of Temperature Kelvin Unit of Amount of Substance Unit of Volume: Mole derived unit (Space or capacity) Liter, milliliter, cubic centimeter

Metric Unit of Volume 10 cm X 10 cm Liter Derived Unit

1000 ? 3 cm = 1 Liter Derived Unit

1000 m. L = 1 Liter ? milliliter = 1 Liter Derived Unit

1 m. L = 1 3 cm ? milliliter = 1 3 cm Derived Unit

1 kilogram What is the mass of 1 liter of pure H 2 O? Mass/Volume relationship

1 liter What is the volume of 1 kg of pure H 2 O? Mass/Volume relationship

1 gram What is the mass of 1 3 cm of pure H 2 O? Mass/Volume relationship

1 3 cm or 1 m. L What is the volume of 1 gram of pure H 2 O? Mass/Volume relationship

273 K Freezing Point of water in the Kelvin scale. Physical Constant

373 K Boiling Point of water in the Kelvin scale. Physical Constant

0 C Freezing Point of water in the centigrade scale. Physical Constant

100 C Boiling Point of water in the centigrade scale. Physical Constant

Another name for freezing point. Ice / water equilibrium

Another name for boiling point. Steam / water equilibrium

100 cm = 1 meter ? cm in 1 meter Conversion Fact

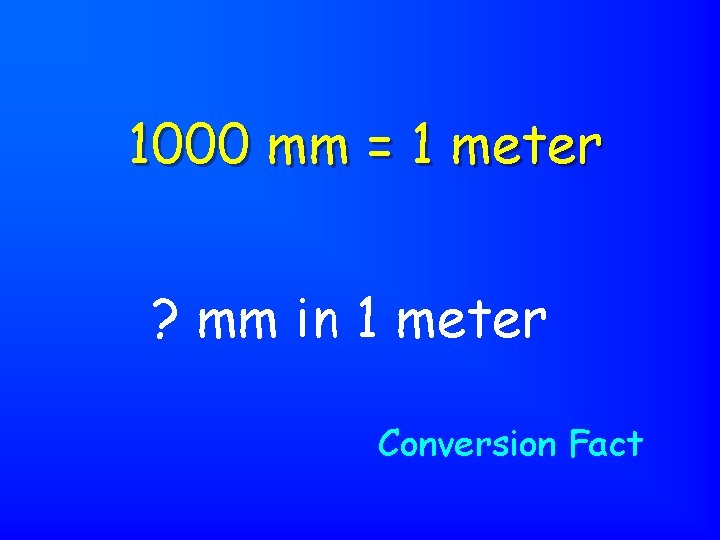
1000 mm = 1 meter ? mm in 1 meter Conversion Fact

1000 m = 1 kilometer ? m in 1 km Conversion Fact

1000 mg = 1 gram ? mg in 1 g Conversion Fact

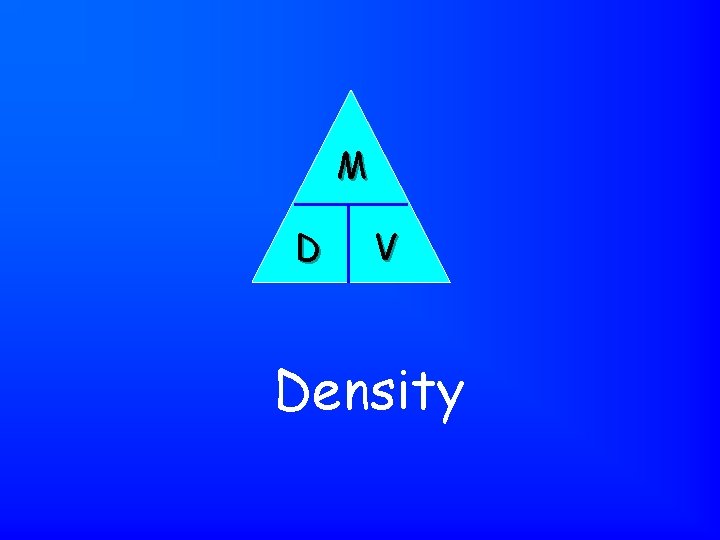
Mass / Volume Density

Describes how matter is packed into space. Density

grams / cm 3 for solids grams / ml for liquids Units of Density

M D V Density

How close a measured value is to an accepted value. Accuracy

How close a series of measurements are to one another. Precision

Low Scatter High Precision

Measured value – Accepted value x 100% Accepted value Percent Error Table T, reference tables

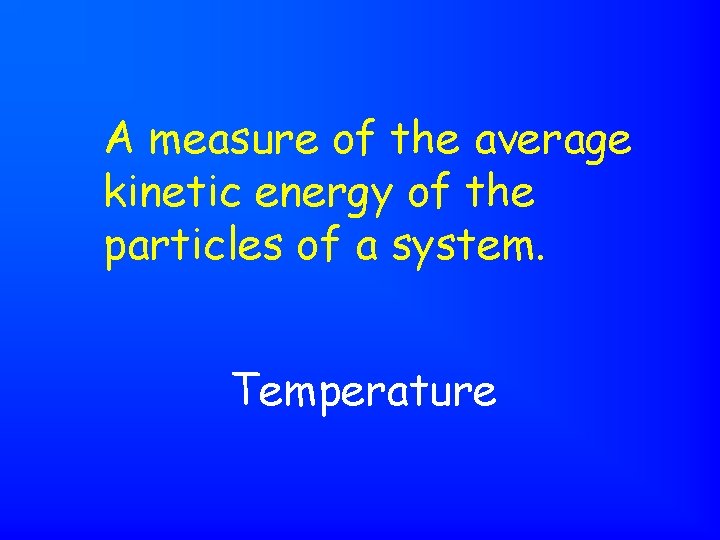
A measure of the average kinetic energy of the particles of a system. Temperature

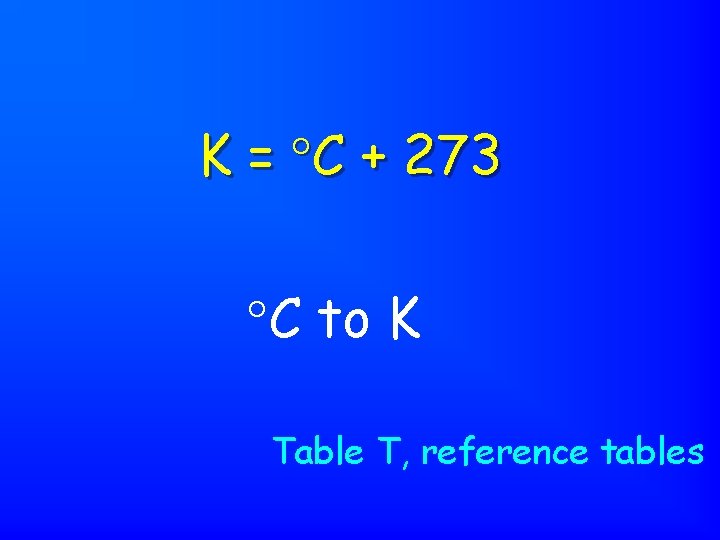
K = C + 273 C to K Table T, reference tables

The number is written as a product of 2 numbers: - a number between 1 & 10 - a power of 10 Scientific Notation
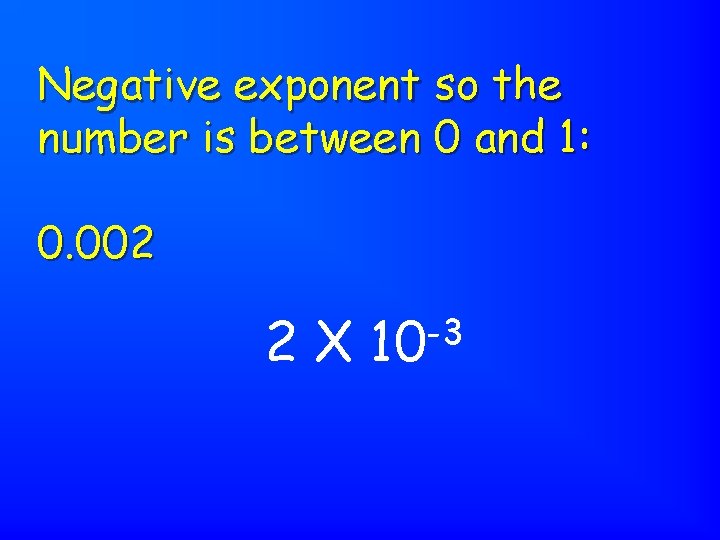
Negative exponent so the number is between 0 and 1: 0. 002 2 X -3 10

Positive exponent so the number is greater than 1: 500 5 X 2 10

3. 45 X The 1 st factor got smaller by a factor of 100. -17 10 So the 2 nd factor must increase by a factor of 100. Convert 345 X 10 -19 to scientific notation.

All known digits plus 1 estimated digit. Significant Figures From the perspective of the person MAKING the measurement.

Decimal Present – Pacific side Start counting at 1 st nonzero # and count until the end of the number. 5400. 145 cm 1 2 34 567 Interpreting someone else’s measurements. 7 sig figs

Decimal Present – Pacific side Start counting at 1 st nonzero # and count until the end of the number. 0. 0175 g 12 3 Interpreting someone else’s measurements. 3 sig figs

Decimal Absent – Atlantic side Start counting at 1 st nonzero # and count until the end of the number. 4855 g 4 321 4 sig figs

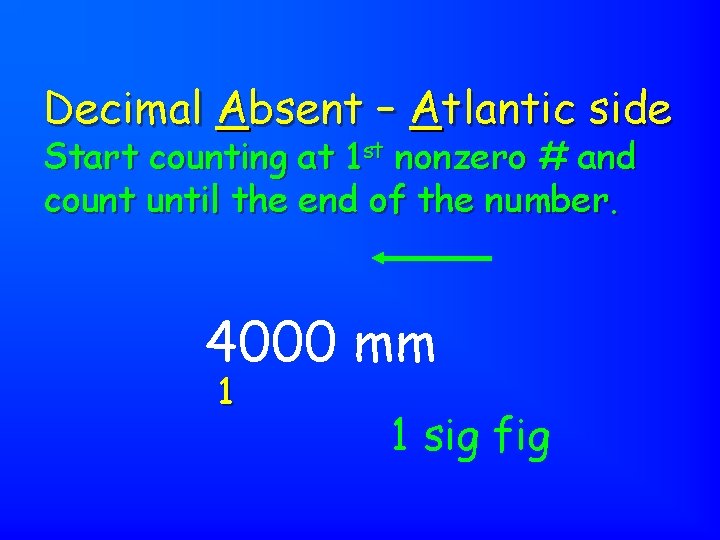
Decimal Absent – Atlantic side Start counting at 1 st nonzero # and count until the end of the number. 4000 mm 1 1 sig fig

Answer has same number of decimal places as the addend with the least number of decimal places. Rule for sig figs in Addition and Subtraction

77. 2 cm is correct! Least # of decimal places ! 28. 0 cm 23. 538 cm + 25. 68 cm 77. 218 cm But what do you report?

Answer has same number of significant figures as the factor with the least number of significant figures. Rule for sig figs in Multiplication & Division

78 m 2 is correct! 2 sig figs 3 sig figs 24 m X 3. 26 m = 78. 24 But what do you report? 2 m

a) 2. 749 g/cm 3 b) 2. 75 g/cm 3 c) 2. 7 g/cm 3 d) 0. 36 cm 3/g 3 sig figs An aluminum cube has a mass of 4. 75 grams. The dimensions of the cube are 1. 2 cm X 1. 2 cm. What is the density of the 2 sf cube? “Volume” = 1. 728 cm 3 “Volume” = 1. 728 cm

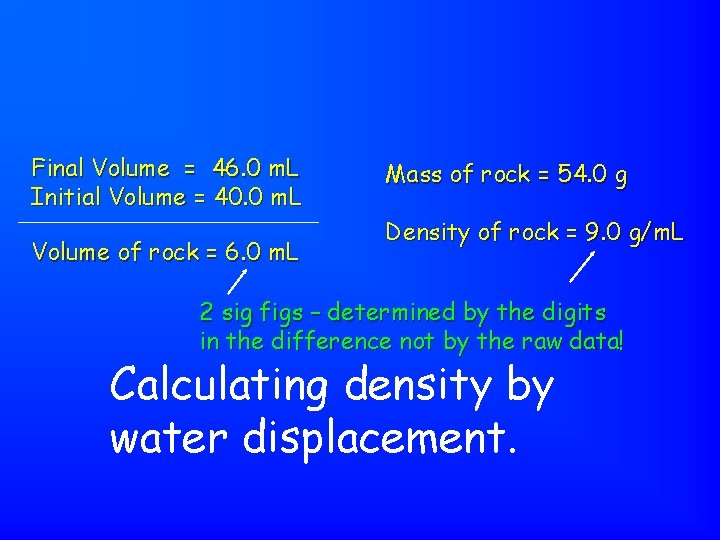
Final Volume = 46. 0 m. L Initial Volume = 40. 0 m. L Volume of rock = 6. 0 m. L Mass of rock = 54. 0 g Density of rock = 9. 0 g/m. L 2 sig figs – determined by the digits in the difference not by the raw data! Calculating density by water displacement.

Molar Mass of Na. Cl = 58. 5 grams. Calculated from P. T. 43. 875 g 1 mole 58. 5 grams = 0. 75000 moles/1. 0 Liter = 0. 75 M 2 sig fig 5 sig figs A solution contains 43. 875 grams of Na. Cl dissolved in 1. 0 Liter of H 2 O. What is the molarity expressed to the correct sig figs?

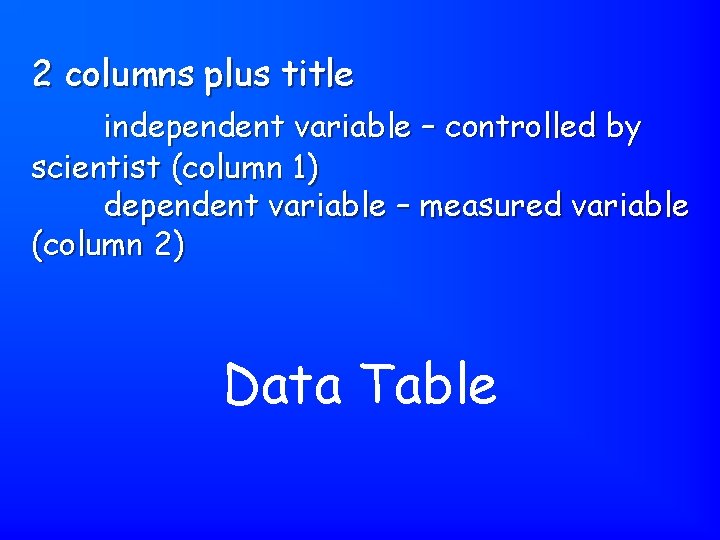
2 columns plus title independent variable – controlled by scientist (column 1) dependent variable – measured variable (column 2) Data Table

2 axes plus title independent variable – controlled by scientist (column 1) – GOES on X-AXIS dependent variable – measured variable (column 2) – GOES on Y-AXIS Graph

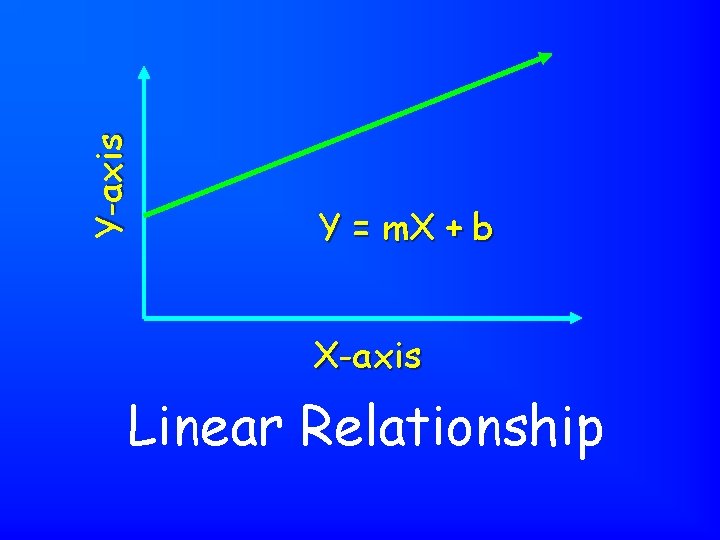
Y-axis Y = m. X + b X-axis Linear Relationship

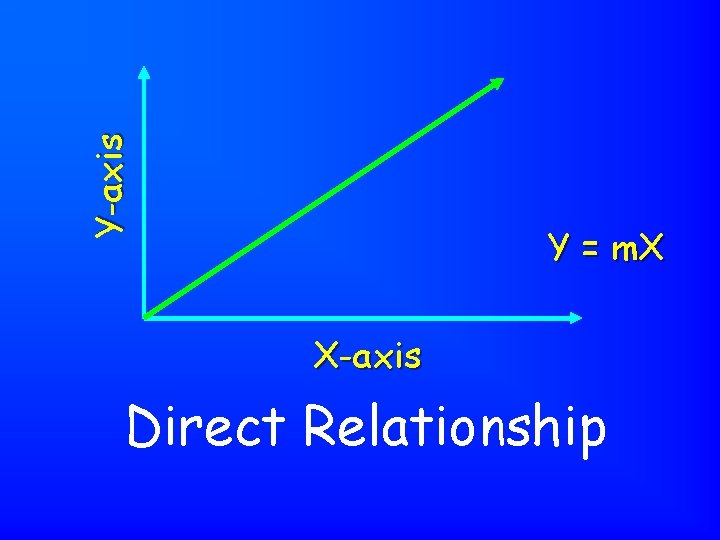
Y-axis Y = m. X X-axis Direct Relationship

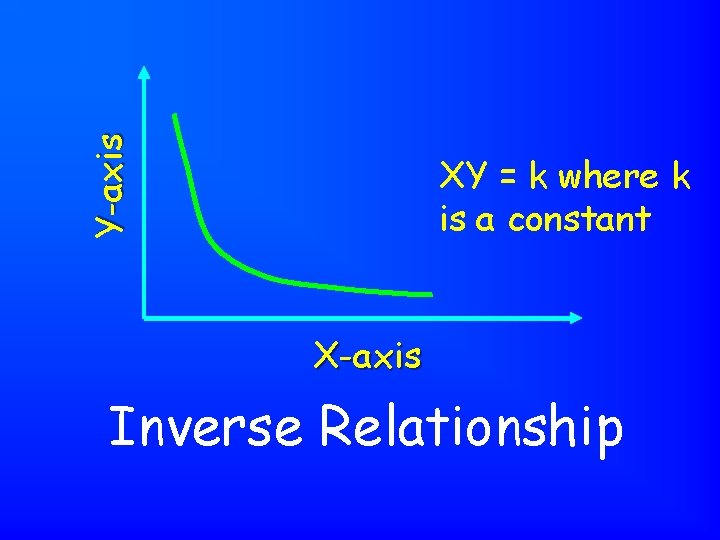
Y-axis XY = k where k is a constant X-axis Inverse Relationship

Y-axis X-axis Graph of a Constant