FLASHBACK Chemical Reactions Types of Chemical Equations Balancing

FLASHBACK! Chemical Reactions Types of Chemical Equations Balancing Chemical Equations Reaction Rates 1

Why should we care about chemical combinations? q Awareness of basic chemistry can save your life! § § q Example: mixing certain cleaning solutions can produce a poisonous gas → Ammonia (Windex) + Bleach ( Clorox) Chloroamine (a poisonous gas) Chemistry is alive in our everyday life: § Ice Packs, Hot Packs, Air Bags in Cars, Foods

Compounds are groupsof elementschemically bonded together 2 types we’ve studied: IONIC – bonding where electrons are gained or lost COVALENT – bonding where electrons areshared

EXOthermic. Reactions A reaction thatreleasesenergy and gives off heat/light/soundresulting in araising of temperature “energy” will be with theproducts side of a chemical equations Examples: glow sticks, fire, lycopodium powder

ENDOthermic A reaction that. ABSORBES energy resulting in. LOWERING of temperature. “energy” will be with the. REACTANTS side of a chemical equations Examples: ANTACID & WATER, PHOTOSYNTHESIS
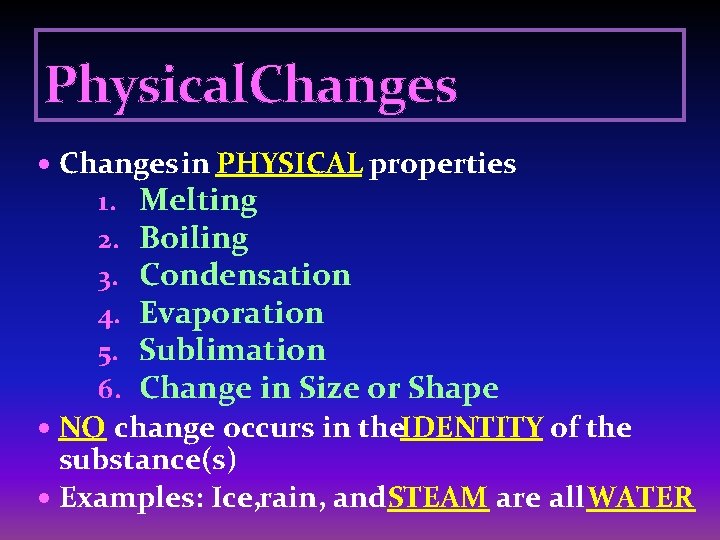
Physical. Changes in PHYSICAL properties 1. 2. 3. 4. 5. 6. Melting Boiling Condensation Evaporation Sublimation Change in Size or Shape NO change occurs in the. IDENTITY of the substance(s) Examples: Ice, rain, and. STEAM are all WATER

Chemical Changes Atoms in the. REACTANTS are REARRAGED to form one or more. DIFFERENT substances OLD chemical BONDS are BROKEN and NEW chemical BONDS are FORMED Changes DO occur in the identity of the compounds since NEW compounds are formed Examples: ØFe and O 2 form RUST (Fe 2 O 3) ØAg and S form TARNISH (Ag 2 S)

Signs of Chemical Changes 1. New substance produced 2. Production of a gas 3. Change in color 4. Change in smell 5. Change in heat or light

Learning. Check #1 Classify each of the following as a PHYSICAL CHANGE (P) or a CHEMICAL CHANGE (C) A. ____ a burning candle B. ____ steam coming up from boiling H 2 O C. ____ toasting a marshmallow D. ____ cutting a pizza E. ____ polishing silver

Solution #1 Classify each of the following as a PHYSICAL CHANGE (P) or a CHEMICAL CHANGE (C) A. _C_ a burning candle B. _P_ steam coming up from boiling H 2 O C. _C _ toasting a marshmallow D. _ P cutting a pizza E. _ C _ polishing silver

Chemical Reaction A process in which. AT LEAST ONE NEW SUBSTANCE IS PRODUCED AS A RESULT OF A CHEMICAL CHANGE. 11

A Chemical Reaction REACTANTS PRODUCTS Draw the contents of these two boxes on your paper

Learning Check #2 A. Use the box drawing to determine, how does the equation indicate a chang in the identity of the reacting substances? B. Does the equation follow the Law of Conservation of Matter? How can you tell?

Solution #2 A. Use the box drawing to determine, how does an equation indicate a change in the identity of the reacting substances? The formulas of the reactants are different than the formulas of the products. B. Does the equation follow the Law of Conservation of Matter? How can you tell? Yes, there are the same number and kinds of symbols (atoms) on both sides of the chemical equation.
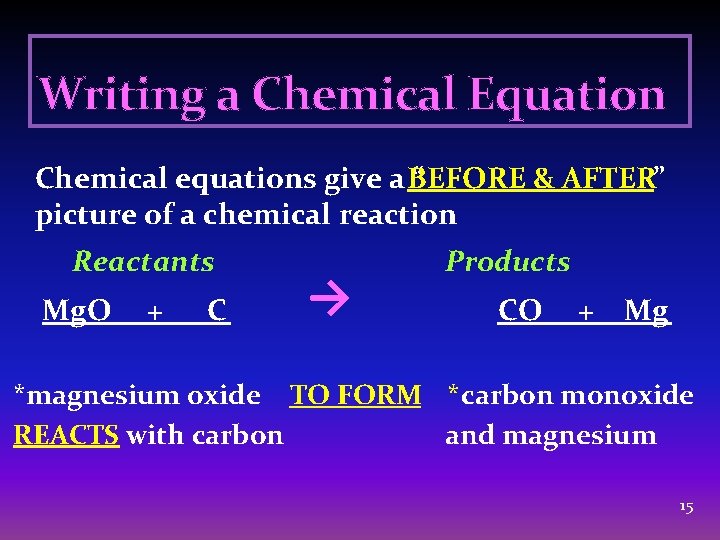
Writing a Chemical Equation Chemical equations give a BEFORE “ & AFTER” picture of a chemical reaction Reactants Mg. O + C → Products CO + Mg *magnesium oxide TO FORM *carbon monoxide REACTS with carbon and magnesium 15

Learning Check #3 12 oz of dough, 4 oz mushrooms, 12 slices pepperoni, 8 oz cheese and 5 oz tomato sauce are used to make a pizza. A. How would you write the recipeabove as an equation?

Solution #3 12 oz of dough, 4 oz mushrooms, 12 slices pepperoni, 8 oz cheese and 5 oz tomato sauce are used to make a pizza. A. How would you write therecipe as an equation? 12 oz dough + 4 oz mshrm + 12 pep + 8 oz chse + 5 oz tom sauce 1 pizza
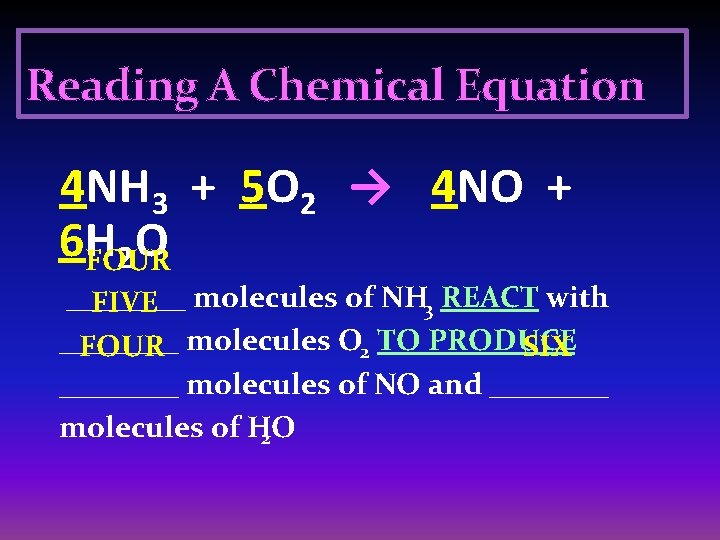
Reading A Chemical Equation 4 NH 3 + 5 O 2 → 4 NO + 6 H O 2 FOUR ____ FIVE molecules of NH 3 REACT with ____ FOUR molecules O 2 TO PRODUCE SIX ____ molecules of NO and ____ molecules of H 2 O

A Balanced Chemical Equation SAME numbers of. EACH TYPE OF ATOM on each side of the equation S → Al 2 S 3 Not Balanced 2 Al + 3 S → Al 2 S 3 Balanced Al + When balancing an equation, NEVER _______change ALWAYS change COEFFICIENTS subscripts; ______________
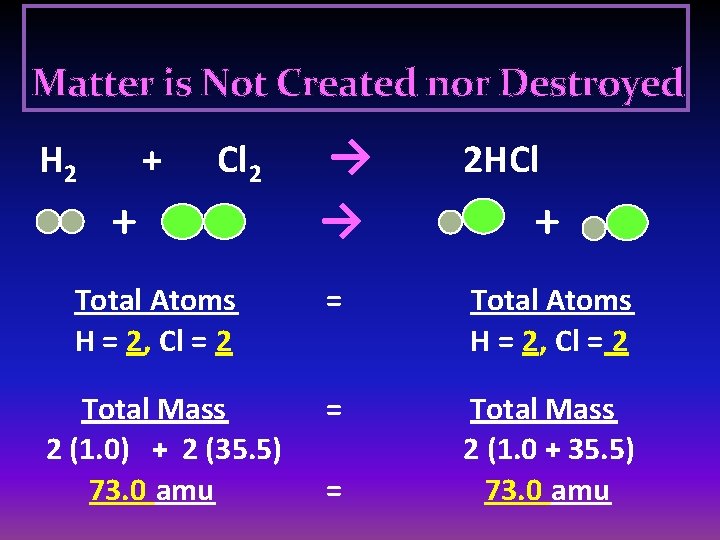
Matter is Not Created nor Destroyed → → 2 HCl Total Atoms H = 2, Cl = 2 = Total Atoms H = 2, Cl = 2 Total Mass 2 (1. 0) + 2 (35. 5) 73. 0 amu = Total Mass 2 (1. 0 + 35. 5) 73. 0 amu H 2 + Cl 2 + = +

Law of Conservation of Mass In any ordinary chemical reaction, MATTER IS NOT CREATED or DESTROYED

Balance Equations with Coefficients Add Coefficients. IN FRONT of compounds to BALANCE the equation 4 NH 3 + 5 O 2 N= 4 H = 12 O = 10 4 NO + 6 H 2 O & & & N= 4 H = 12 O = 10
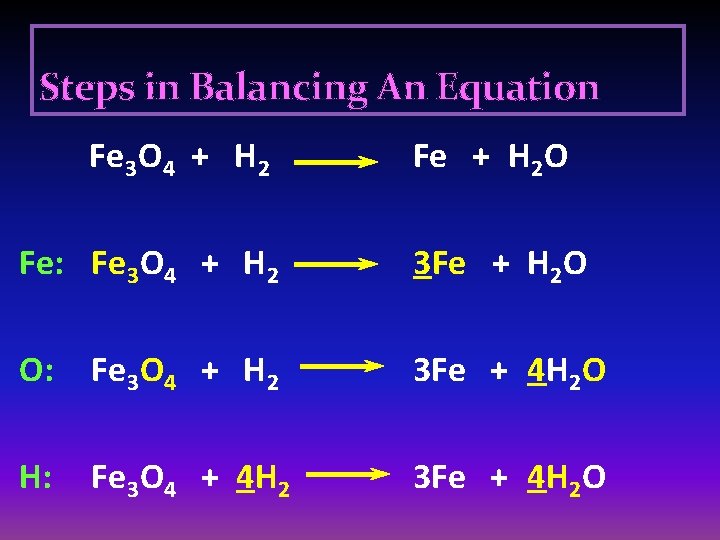
Steps in Balancing An Equation Fe 3 O 4 + H 2 Fe + H 2 O Fe: Fe 3 O 4 + H 2 3 Fe + H 2 O O: Fe 3 O 4 + H 2 3 Fe + 4 H 2 O H: Fe 3 O 4 + 4 H 2 3 Fe + 4 H 2 O
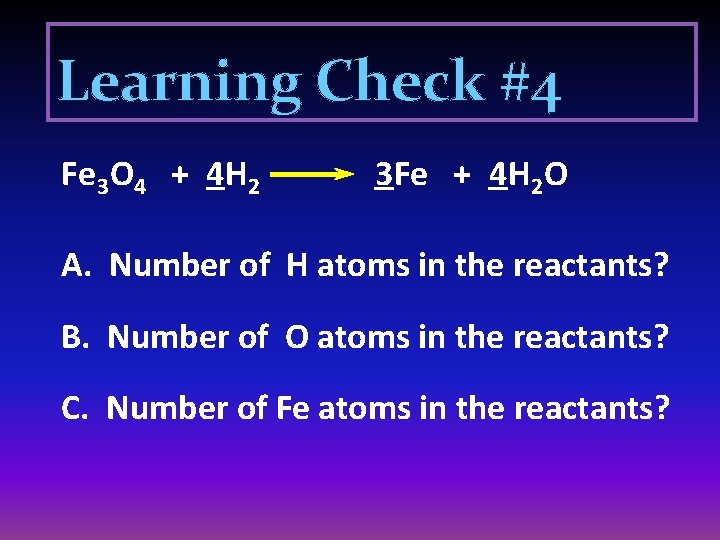
Learning Check #4 Fe 3 O 4 + 4 H 2 3 Fe + 4 H 2 O A. Number of H atoms in the reactants? B. Number of O atoms in the reactants? C. Number of Fe atoms in the reactants?

Solution #4 Fe 3 O 4 + 4 H 2 3 Fe + 4 H 2 O A. Number of H atoms in the reactants? 8 B. Number of O atoms in the reactants? 4 C. Number of Fe atoms in the reactants? 3
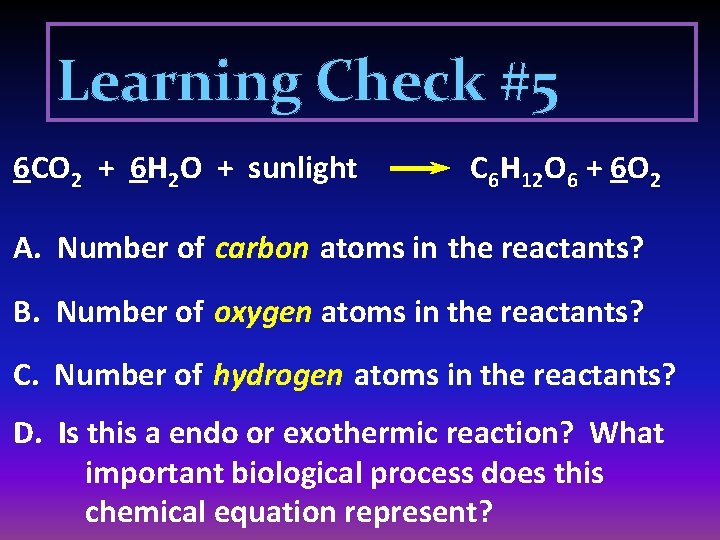
Learning Check #5 6 CO 2 + 6 H 2 O + sunlight C 6 H 12 O 6 + 6 O 2 A. Number of carbon atoms in the reactants? B. Number of oxygen atoms in the reactants? C. Number of hydrogen atoms in the reactants? D. Is this a endo or exothermic reaction? What important biological process does this chemical equation represent?
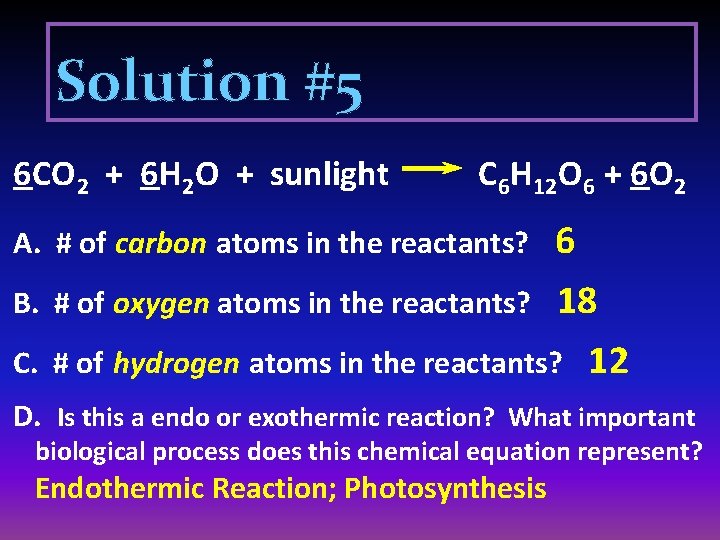
Solution #5 6 CO 2 + 6 H 2 O + sunlight C 6 H 12 O 6 + 6 O 2 6 # of oxygen atoms in the reactants? 18 # of hydrogen atoms in the reactants? 12 A. # of carbon atoms in the reactants? B. C. D. Is this a endo or exothermic reaction? What important biological process does this chemical equation represent? Endothermic Reaction; Photosynthesis
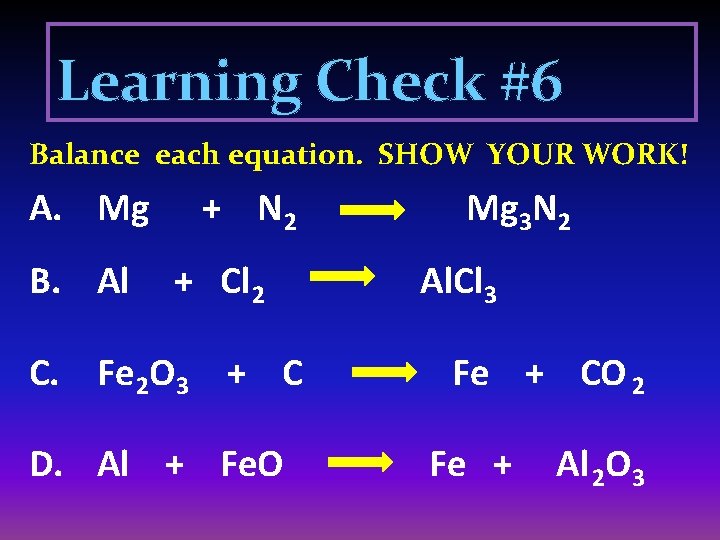
Learning Check #6 Balance each equation. SHOW YOUR WORK! A. Mg B. Al + N 2 + Cl 2 C. Fe 2 O 3 + C D. Al + Fe. O Mg 3 N 2 Al. Cl 3 Fe + CO 2 Fe + Al 2 O 3
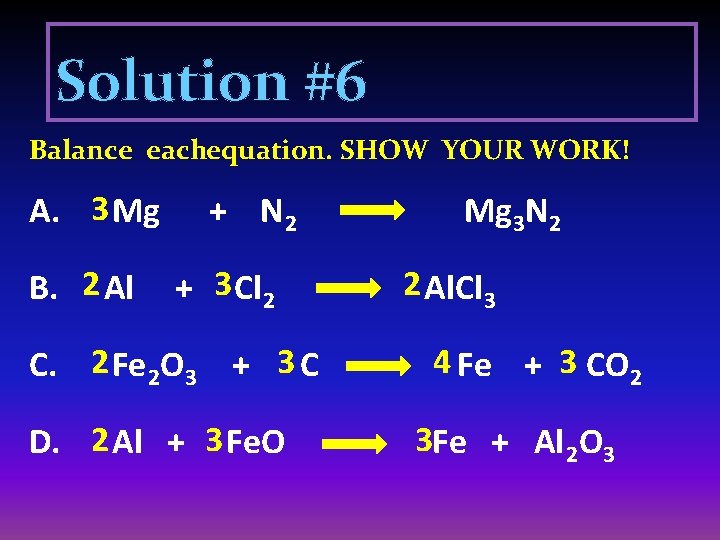
Solution #6 Balance eachequation. SHOW YOUR WORK! A. 3 Mg B. 2 Al + N 2 + 3 Cl 2 C. 2 Fe 2 O 3 + 3 C D. 2 Al + 3 Fe. O Mg 3 N 2 2 Al. Cl 3 4 Fe + 3 CO 2 3 Fe + Al 2 O 3
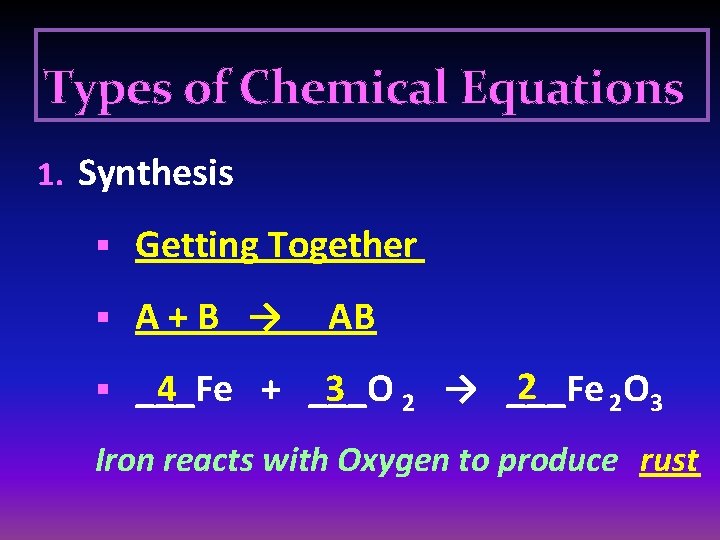
Types of Chemical Equations 1. Synthesis § Getting Together § A+B → AB 2 4 3 2 → ___Fe § ___Fe + ___O 2 O 3 Iron reacts with Oxygen to produce rust
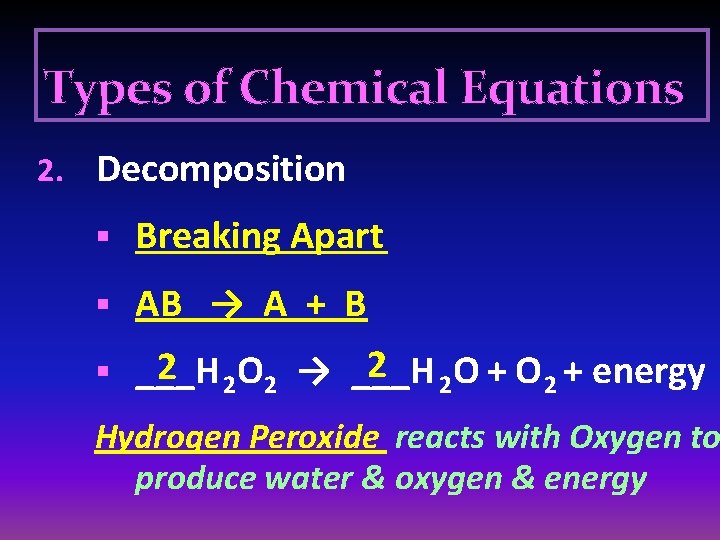
Types of Chemical Equations 2. Decomposition § Breaking Apart § AB → A + B 2 2 O + O 2 + energy 2 2 O 2 → ___H § ___H Hydrogen Peroxide reacts with Oxygen to produce water & oxygen & energy
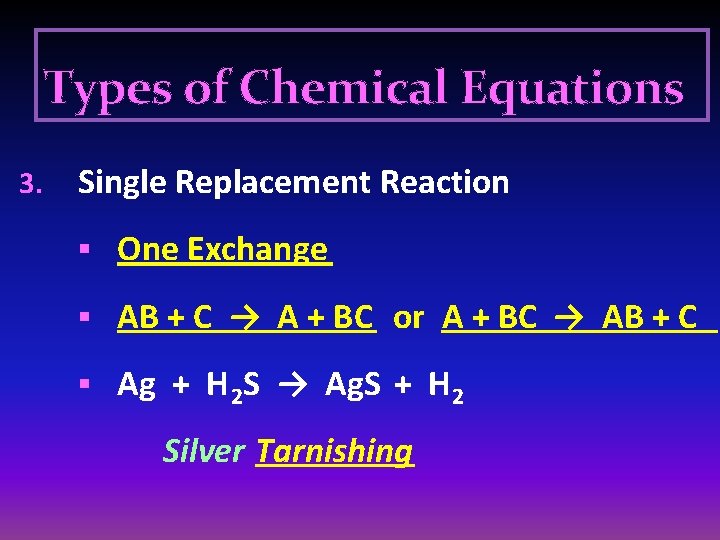
Types of Chemical Equations 3. Single Replacement Reaction § One Exchange § AB + C → A + BC or A + BC → AB + C § Ag + H 2 S → Ag. S + H 2 Silver Tarnishing
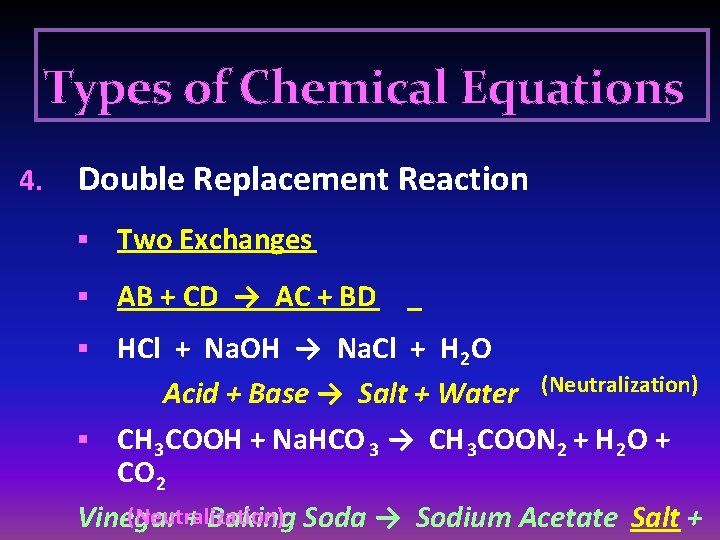
Types of Chemical Equations 4. Double Replacement Reaction § Two Exchanges § AB + CD → AC + BD HCl + Na. OH → Na. Cl + H 2 O Acid + Base → Salt + Water (Neutralization) § CH 3 COOH + Na. HCO 3 → CH 3 COON 2 + H 2 O + CO 2 (Neutralization) Vinegar + Baking Soda → Sodium Acetate Salt + §
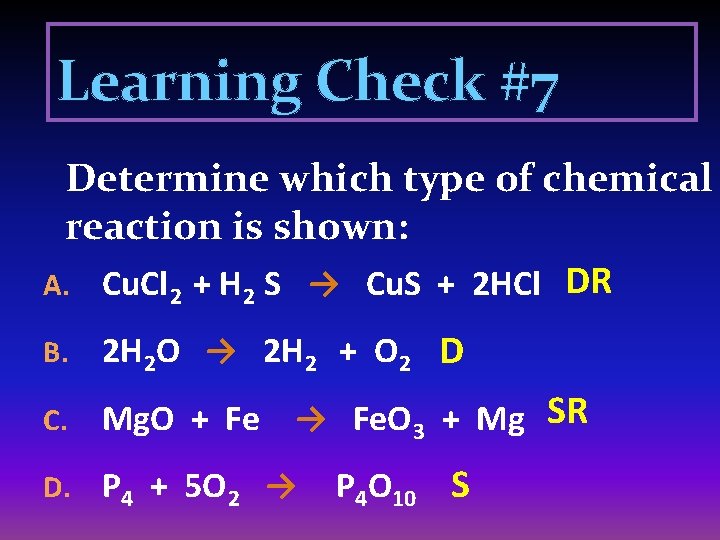
Learning Check #7 Determine which type of chemical reaction is shown: A. Cu. Cl 2 + H 2 S → Cu. S + 2 HCl DR B. 2 H 2 O → 2 H 2 + O 2 D C. Mg. O + Fe → Fe. O 3 + Mg SR D. P 4 + 5 O 2 → P 4 O 10 S

E. Ag + H 2 S F. Fe 3 O 4 + 4 H 2 G. 3 Mg H. 3 Al I. Ag. S + H 2 SR → 3 Fe + 4 H 2 O SR → + N 2 → + 3 Cl 2 → HCl + Na. OH → Mg 3 N 2 S 2 Al. Cl 3 S Na. Cl + H 2 O DR 2 Fe 2 O 3 + 3 C → 4 Fe + 3 CO 2 SR K. 2 Al + 3 Fe. O → 3 Fe + Al 2 O 3 SR J. L. 2 Fe 2 O 3 → 4 Fe + 3 O 2 D

Chemical Reaction Rates • “Reaction Rates” refers to HOW FAST A REACTION OCCURS
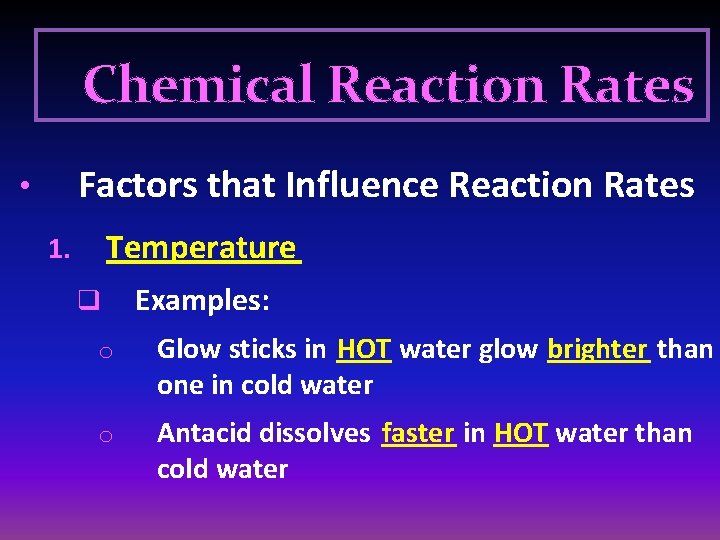
Chemical Reaction Rates Factors that Influence Reaction Rates • Temperature 1. q Examples: o Glow sticks in HOT water glow brighter than one in cold water o Antacid dissolves faster in HOT water than cold water
Chemical Reaction Rates Factors that Influence Reaction Rates • Surface Area 2. q Refers to how much of the outside of a substance is exposed q The greater surface area, the faster the reaction q Examples: o Lycopodium Powder o Headache medicine reacts faster in powder form than solid form (ex. Goodie Powder versus Tylenol Pill)

Chemical Reaction Rates Factors that Influence Reaction Rates • Concentration 3. q q q o Refers to how much of a substance there is The greater concentration of a reactant, the faster the reaction Example: Vinegar & Baking Soda in Beaker – more vinegar produces a faster reaction

Chemical Reaction Rates Factors that Influence Reaction Rates • Presence of a Catalyst 4. q Refers to a substance that speeds up a reaction but is NOT used up by the reaction q The catalyst can be recovered unchanged , and reused indefinitely q Examples: o Yeast in our “chemiluminescent elephant’s toothpaste” reaction o Enzymes found in animals that speed up digestion

Chemical Reaction Rates Factors that Influence Reaction Rates • Presence of an Inhibitor 5. q q o o o Refers to something that slows down or stops a chemical reaction Examples: Why do we cover our left over foods? Why do we even put food in the fridge? Why do we use food preservatives like

Learning Check #8 List 4 ways to make a reaction occur faster (increasethe speed): 1. 2. 3. 4.

Learning Check #9 List 4 ways to make a reaction occur slower (decreasethe speed): 1. 2. 3. 4.

Learning Check #10 See Study Guide From Chapter 7 Test
- Slides: 44