FLAME PHOTOMETRY PRINCIPLE Sample solution sprayed into flame










- Slides: 17

FLAME PHOTOMETRY

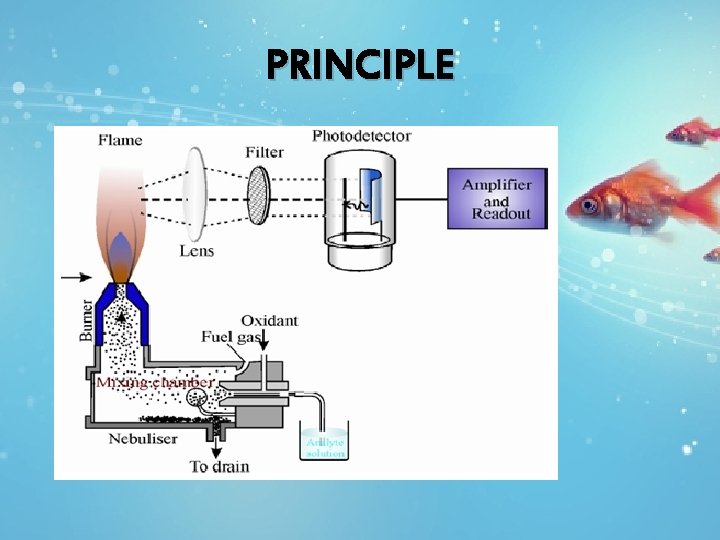
PRINCIPLE

Sample solution sprayed into flame Solvent in droplets evaporated Solid residue left Converts to gaseous atoms(neutral atoms) Neutral atoms exited Emits color radiation to come back to ground state

When a metallic salt solution is aspirated into path of flame, following events takes place 1) Vaporization: The solvent is vaporized leaving particles of solid salt. 2) Atomization : The salt is vaporized and converted into free neutral gaseous atoms or radicals 3) Excitation: Some of these atoms are excited by thermal energy of the flame to higher energy levels.

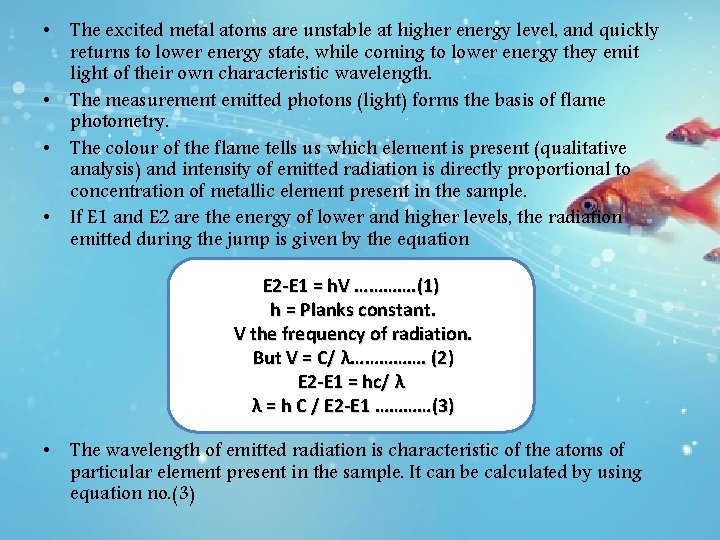
• The excited metal atoms are unstable at higher energy level, and quickly returns to lower energy state, while coming to lower energy they emit light of their own characteristic wavelength. • The measurement emitted photons (light) forms the basis of flame photometry. • The colour of the flame tells us which element is present (qualitative analysis) and intensity of emitted radiation is directly proportional to concentration of metallic element present in the sample. • If E 1 and E 2 are the energy of lower and higher levels, the radiation emitted during the jump is given by the equation E 2 -E 1 = h. V …………. (1) h = Planks constant. V the frequency of radiation. But V = C/ λ……………. (2) E 2 -E 1 = hc/ λ λ = h C / E 2 -E 1 …………(3) • The wavelength of emitted radiation is characteristic of the atoms of particular element present in the sample. It can be calculated by using equation no. (3)

THEORY N* /No= Ae E/ k. T • • • N* = The number of excited atoms. No= The number of atoms remain in ground state. A = Constant of a particular element. E= Difference in the energy level of two states. K= Boltzman constant equal to R/N T= Temperature of flame in Kelvin

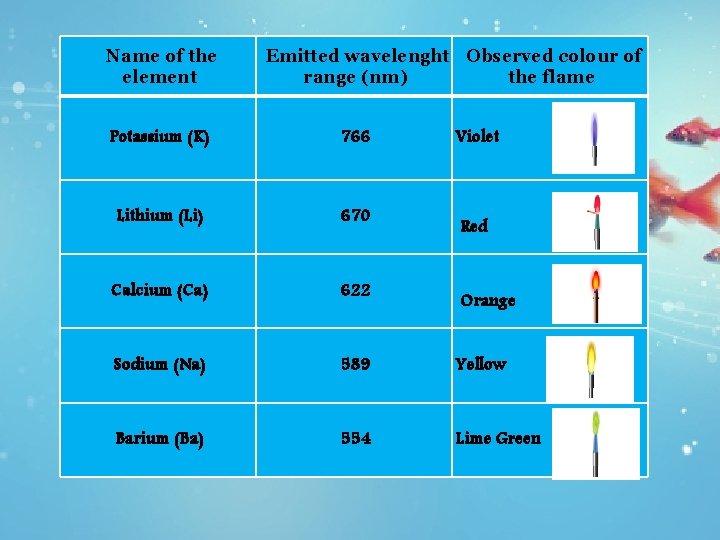
Name of the element Emitted wavelenght Observed colour of range (nm) the flame Potassium (K) 766 Violet Lithium (Li) 670 Calcium (Ca) 622 Sodium (Na) 589 Yellow Barium (Ba) 554 Lime Green Red Orange

INSTRUMENTATION The basic components for flame photometer are as follows: • Burner(source) • Mirrors • Monochromators • Filter • Detector

1)BURNERS • • ü ü ü BURNERS used in the flame photometry should have following properties : The flame should have ability to evaporate the solvent to give a residue. The flame should convert this residue into gases state atoms and finally to individual atoms. The effect of flame is depends upon the temperature of flame and This temperature can be monitored by following methods : Fuel to air ratio Type of solvent for preparing sample solution. Amount of solvent which is entering into the flame. Type of burner used in flame photometer.

MECKER BURNER ü This burner was used earlier and employed natural gas and oxygen. ü Produces relatively low temp. and low excitation energies. ü This are best used for ALKALI metals only. ü Now-a-days it is not used.

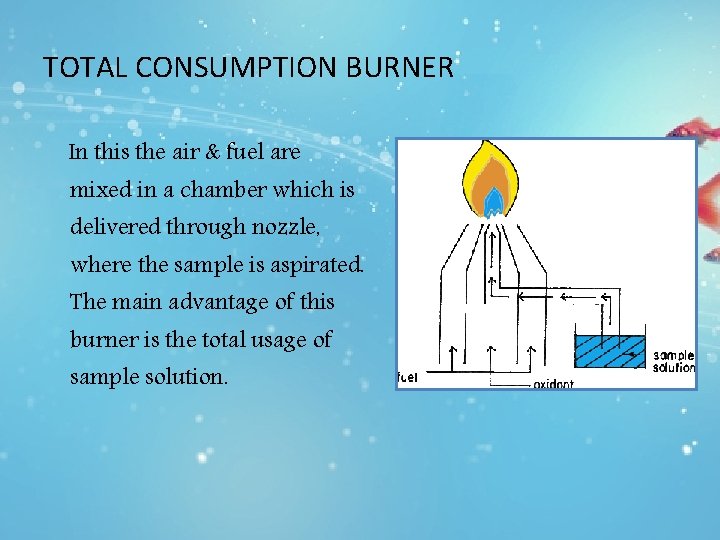
TOTAL CONSUMPTION BURNER In this the air & fuel are mixed in a chamber which is delivered through nozzle, where the sample is aspirated. The main advantage of this burner is the total usage of sample solution.

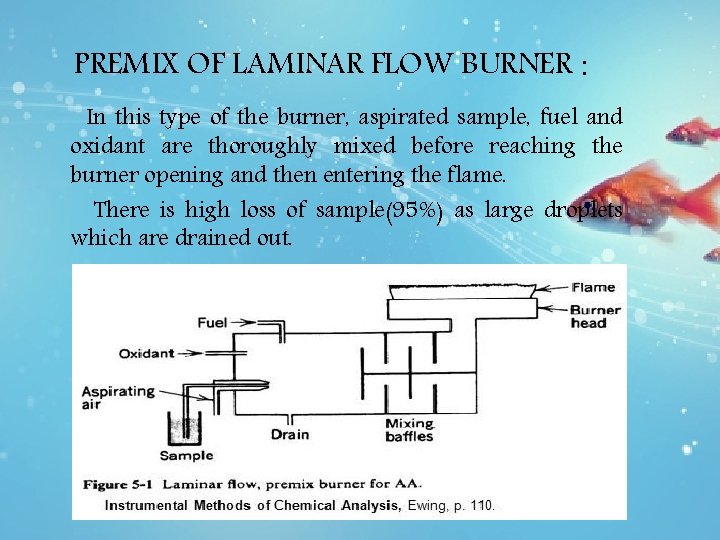
PREMIX OF LAMINAR FLOW BURNER : In this type of the burner, aspirated sample, fuel and oxidant are thoroughly mixed before reaching the burner opening and then entering the flame. There is high loss of sample(95%) as large droplets which are drained out.

LUNDERGARTH BURNER In this sample & air mixed in chamber, this mixed composition send to fuel nozzle where it burn.

2)MIRRORS : ü The radiation from the flame is emitted in all the directions in space. ü Much of the radiation is lost and loss of signal results. ü A mirror is located behind the burner to reflect the radiation back to the entrance slit of the monochromator. ü The reflecting surface of the mirror is front-faced. These mirrors are very easily scratched when subjected to chemical attack ü e. g. acid vapors. Great care should be taken to protect them from corrosive atmosphere.

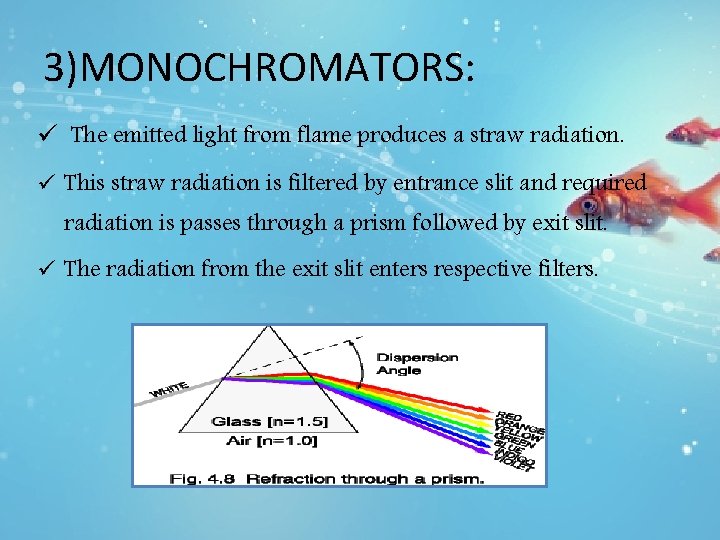
3)MONOCHROMATORS: ü The emitted light from flame produces a straw radiation. ü This straw radiation is filtered by entrance slit and required radiation is passes through a prism followed by exit slit. ü The radiation from the exit slit enters respective filters.

4)FILTERS They are different filters are available based on the ions to be detected like Na, K, Ca, Li, Mg filters etc. These filters allow the specified radiations to the detectors. 5) DETECTORS The function of a detector is to measure the intensity of radiation falling on it. Photo emissive cells or photomultiplier tubes are commonly employed

APPLICATIONS OF FLAME PHOTOMETRY QUALITATIVE APPLICATIONS: ü Some of these elements can be detected visually by the color in the flame, e. g. sodium produces yellow flame. ü The best method is to use flame photometer and when a radiation of the characteristic wavelength is detected, it will indicate the presence of the metal in the sample. ü This is done by peak matching technique. ü Flame photometry is used to determine the concentration of various soil components. ü For agricultural purposes, an analysis of a proper mixture of surface soil and subsoil is carried out to determine the fertilizer requirement of the soil. ü In clinical chemistry, it is very important to determine the concentration of sodium and potassium ions in body fluids since their ratio controls the action of muscles including the heart. ü This is achieved by diluting the blood serum and aspiration into the flame. Food and drinks are analyzed for alkali metals.