Five Types of Reactions 1 Synthesis 2 Decomposition


- Slides: 15


Five Types of Reactions 1. Synthesis 2. Decomposition 3. Single Replacement 4. Double Replacement 5. Combustion

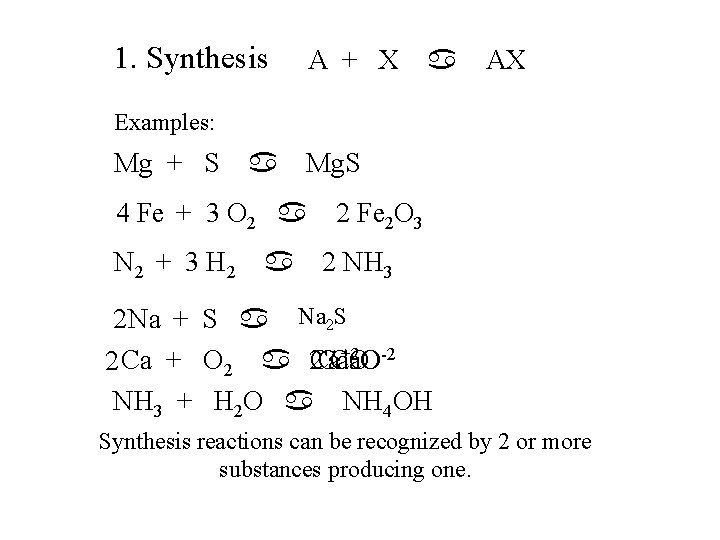
1. Synthesis A + X a AX Examples: Mg + S a Mg. S 4 Fe + 3 O 2 a N 2 + 3 H 2 a 2 Fe 2 O 3 2 NH 3 -2 2 Na + S a Na+1 2 SS +2 O-2 Ca. O 2 Ca + O 2 a 2 Ca NH 3 + H 2 O a NH 4 OH Synthesis reactions can be recognized by 2 or more substances producing one.

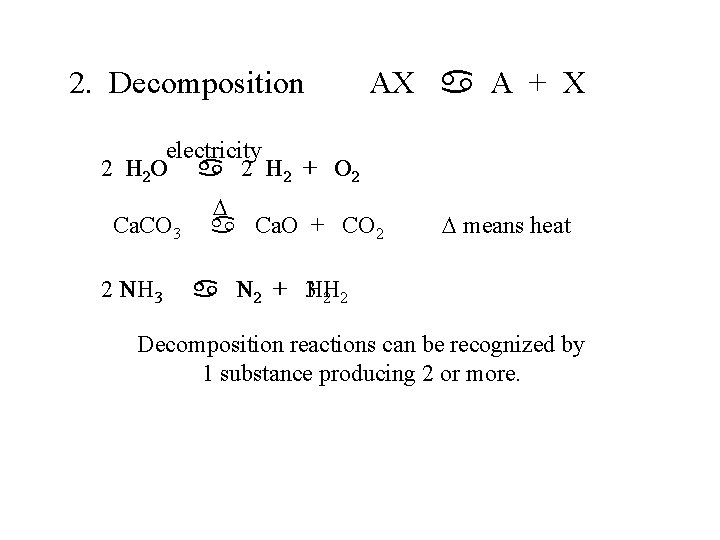
2. Decomposition AX a A + X electricity 2 H 2 O a 2 H 2 + O 2 Ca. CO 3 2 NH 3 D a Ca. O + CO 2 D means heat a N 2 + 3 HH 2 2 Decomposition reactions can be recognized by 1 substance producing 2 or more.

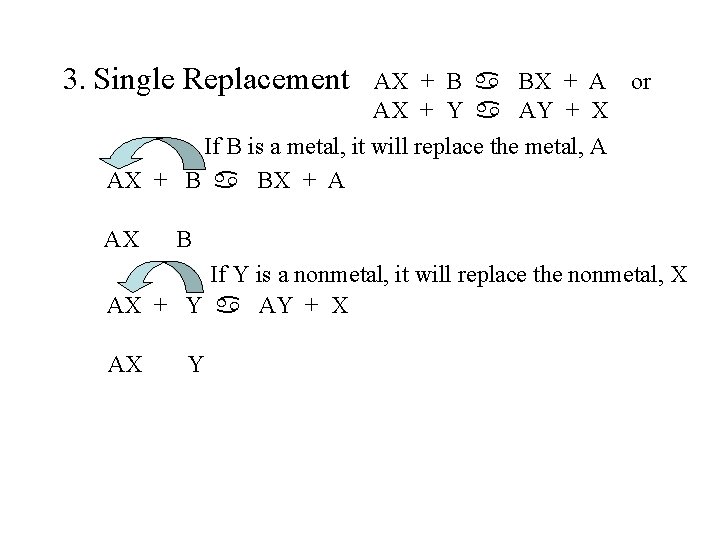
3. Single Replacement AX + B a BX + A or AX + Y a AY + X If B is a metal, it will replace the metal, A AX + B a BX + A AX B If Y is a nonmetal, it will replace the nonmetal, X AX + Y a AY + X AX Y

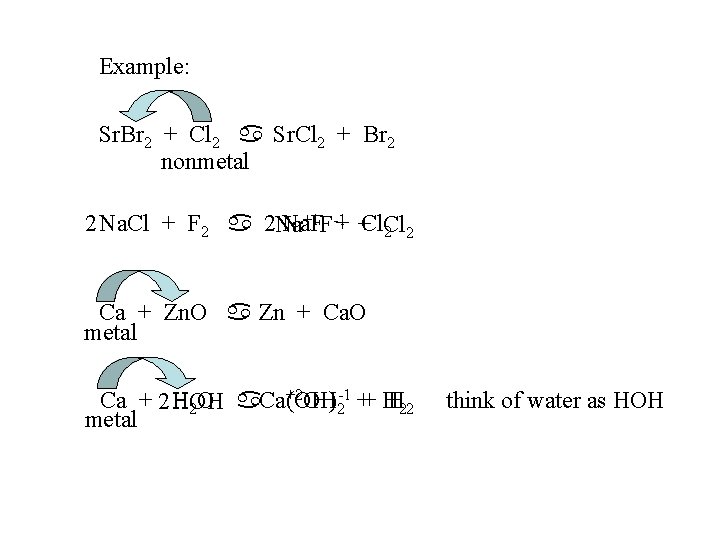
Example: Sr. Br 2 + Cl 2 a Sr. Cl 2 + Br 2 nonmetal +1 F-1 2 Na. Cl + F 2 a 2 Na Na. F + +Cl. Cl 2 2 Ca + Zn. O a Zn + Ca. O metal +2 OH-1 ++ H Ca + 2 HOH H 22 H 2 O a. Ca Ca(OH) 2 metal think of water as HOH

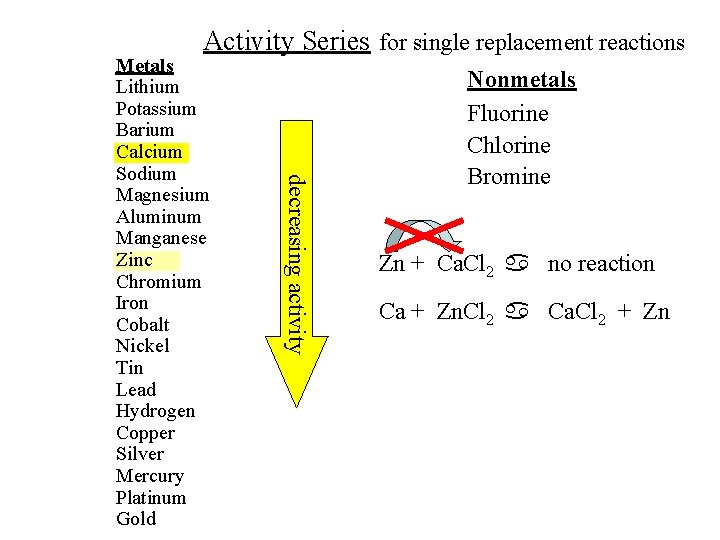
Some replacement reactions are not possible. In order for an element to be replaced, it has to be less active than the element that is replacing it. For example, the reaction Zn + Ca. Cl 2 a no reaction Zn is not more active than Ca. So it cannot replace it.

Activity Series for single replacement reactions decreasing activity Metals Lithium Potassium Barium Calcium Sodium Magnesium Aluminum Manganese Zinc Chromium Iron Cobalt Nickel Tin Lead Hydrogen Copper Silver Mercury Platinum Gold Nonmetals Fluorine Chlorine Bromine Zn + Ca. Cl 2 a no reaction Ca + Zn. Cl 2 a Ca. Cl 2 + Zn

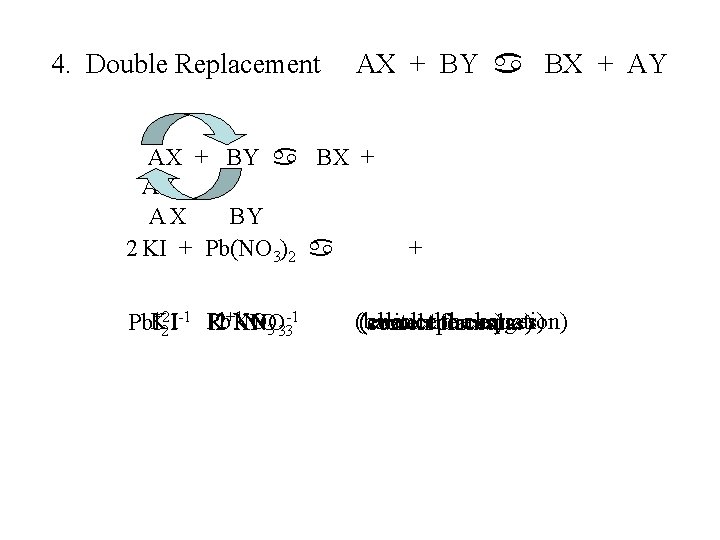
4. Double Replacement AX + BY a BX + AY AX BY 2 KI + Pb(NO 3)2 a +2 I-1 Pb. NO Pb. IK Pb K 2+1 KNO NO 3 33 -1 2 + (balance (check thecharges) equation) (switchthe (correct places) formulas)

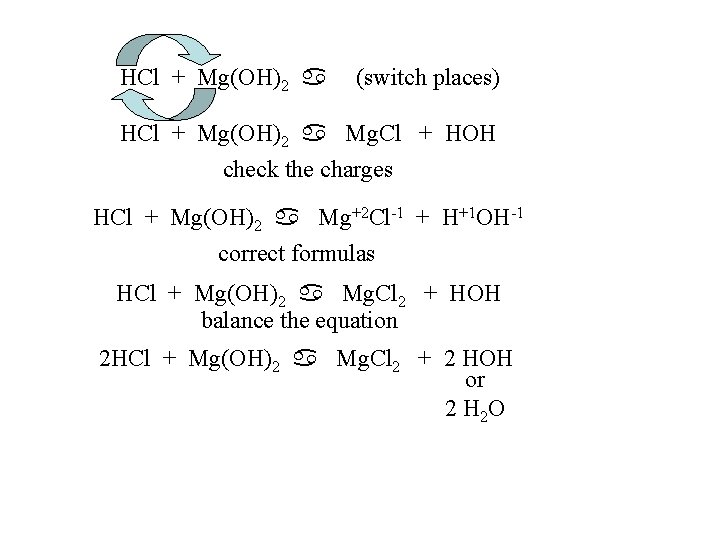
HCl + Mg(OH)2 a (switch places) HCl + Mg(OH)2 a Mg. Cl + HOH check the charges HCl + Mg(OH)2 a Mg+2 Cl-1 + H+1 OH-1 correct formulas HCl + Mg(OH)2 a Mg. Cl 2 + HOH balance the equation 2 HCl + Mg(OH)2 a Mg. Cl 2 + 2 HOH or 2 H 2 O

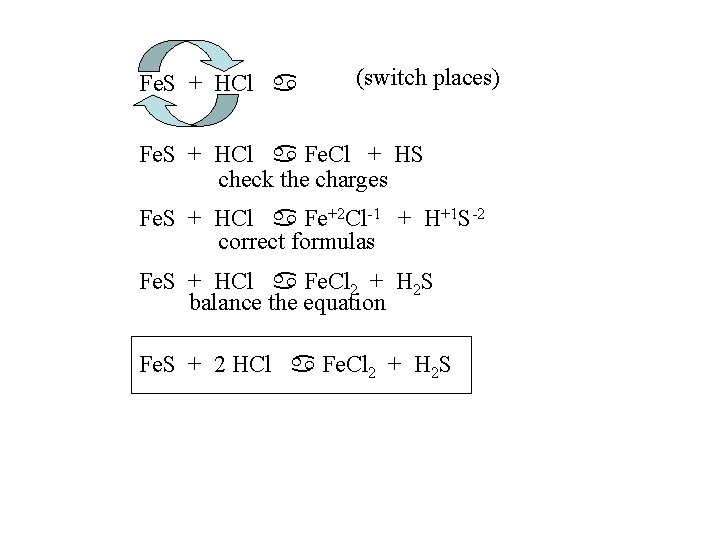
Fe. S + HCl a (switch places) Fe. S + HCl a Fe. Cl + HS check the charges Fe. S + HCl a Fe+2 Cl-1 + H+1 S-2 correct formulas Fe. S + HCl a Fe. Cl 2 + H 2 S balance the equation Fe. S + 2 HCl a Fe. Cl 2 + H 2 S

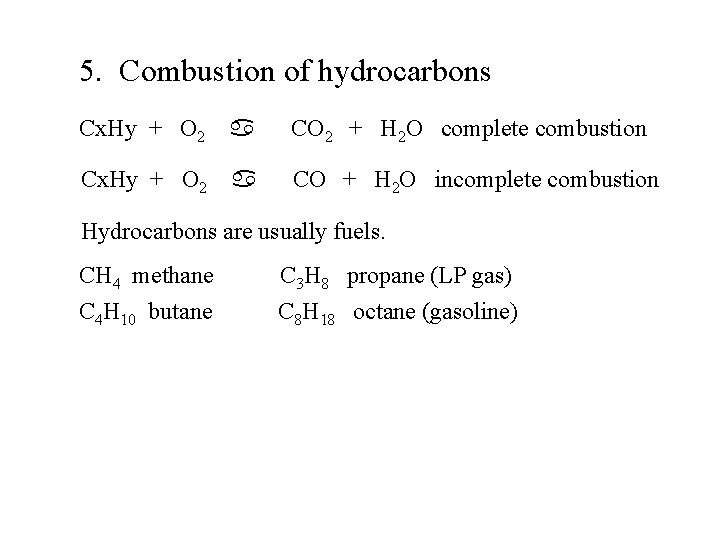
5. Combustion of hydrocarbons Cx. Hy + O 2 a CO 2 + H 2 O complete combustion Cx. Hy + O 2 a CO + H 2 O incomplete combustion Hydrocarbons are usually fuels. CH 4 methane C 4 H 10 butane C 3 H 8 propane (LP gas) C 8 H 18 octane (gasoline)

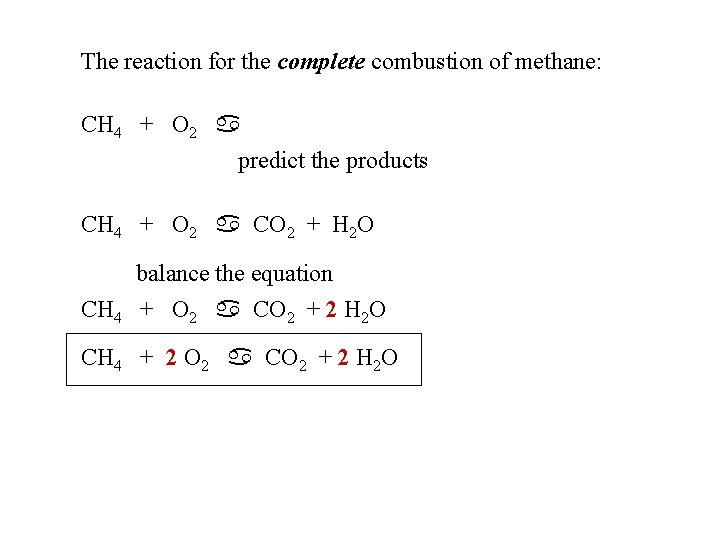
The reaction for the complete combustion of methane: CH 4 + O 2 a predict the products CH 4 + O 2 a CO 2 + H 2 O balance the equation CH 4 + O 2 a CO 2 + 2 H 2 O CH 4 + 2 O 2 a CO 2 + 2 H 2 O

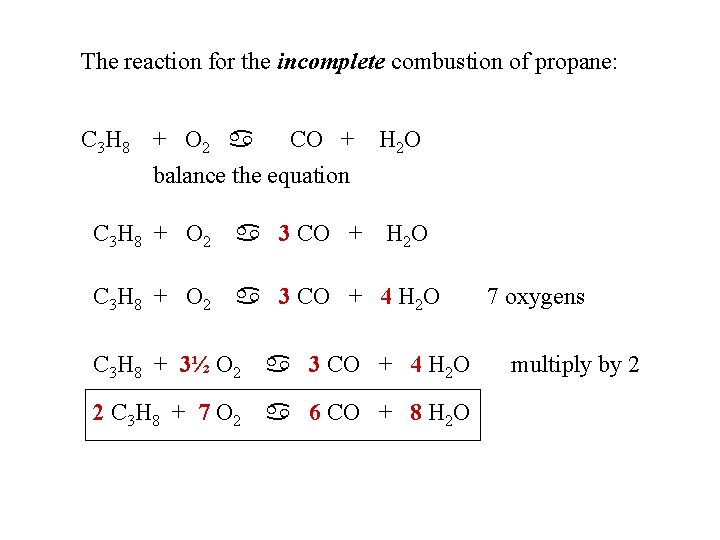
The reaction for the incomplete combustion of propane: C 3 H 8 + O 2 a CO + H 2 O balance the equation C 3 H 8 + O 2 a 3 CO + H 2 O C 3 H 8 + O 2 a 3 CO + 4 H 2 O C 3 H 8 + 3½ O 2 a 3 CO + 4 H 2 O 2 C 3 H 8 + 7 O 2 a 6 CO + 8 H 2 O 7 oxygens multiply by 2

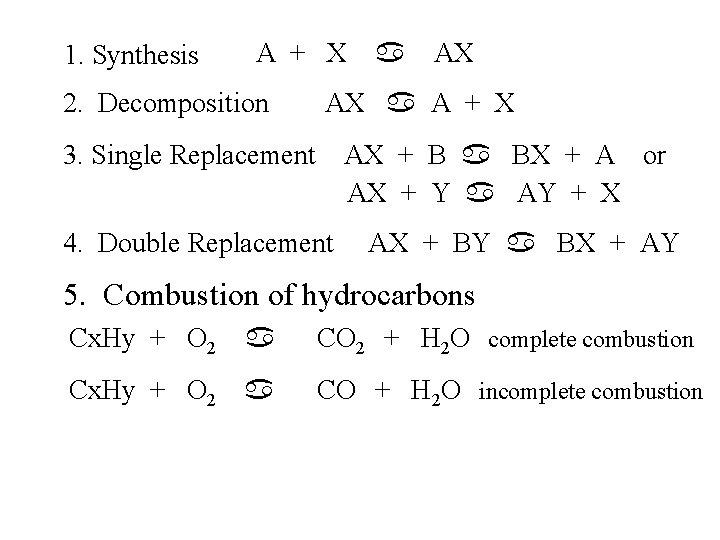
1. Synthesis A + X 2. Decomposition a AX AX a A + X 3. Single Replacement AX + B a BX + A or AX + Y a AY + X 4. Double Replacement AX + BY a BX + AY 5. Combustion of hydrocarbons Cx. Hy + O 2 a CO 2 + H 2 O complete combustion Cx. Hy + O 2 a CO + H 2 O incomplete combustion