Five Basic Geometries Linear Trigonal Tetrahedral Octahedral Trigonal
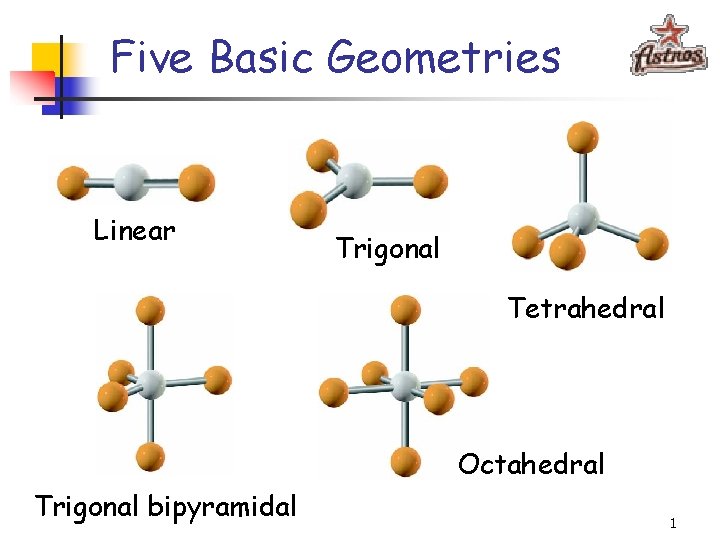
Five Basic Geometries Linear Trigonal Tetrahedral Octahedral Trigonal bipyramidal 1

Se. F 6, IF 5, and Xe. F 4 2
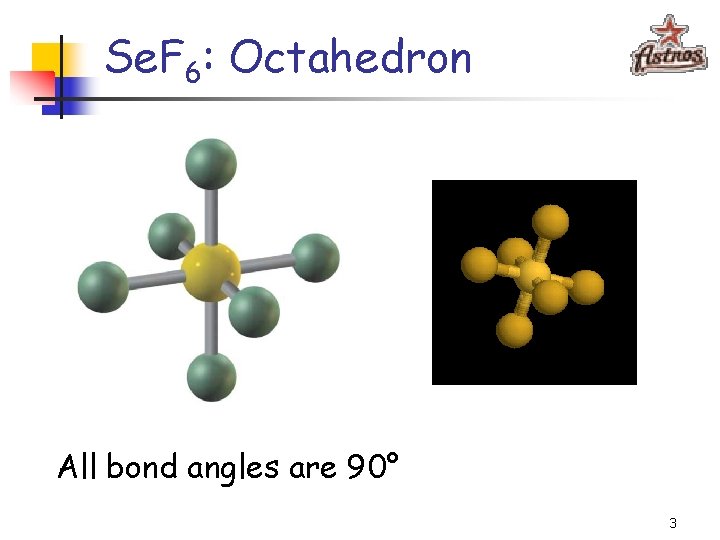
Se. F 6: Octahedron All bond angles are 90° 3
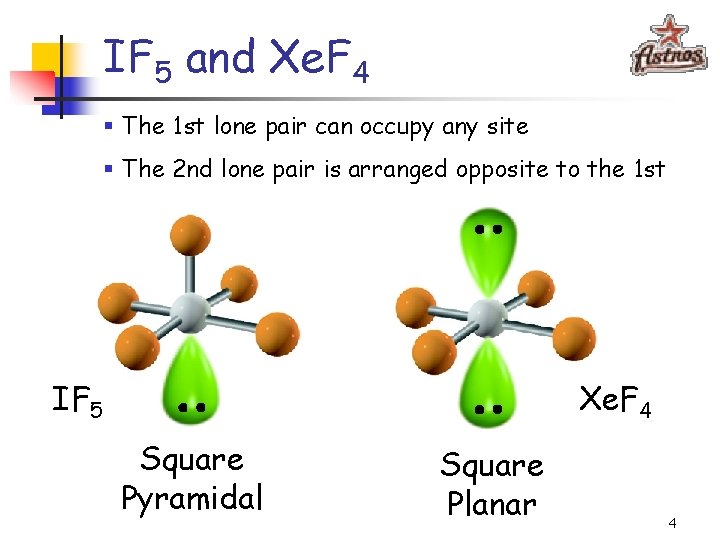
IF 5 and Xe. F 4 § The 1 st lone pair can occupy any site § The 2 nd lone pair is arranged opposite to the 1 st IF 5 Xe. F 4 Square Pyramidal Square Planar 4

Octahedral Electronic Geometry n If lone pairs are incorporated into the octahedral structure, there are 2 possible new shapes § 1 lone pair – Square pyramid § 2 lone pairs – Square planar 5
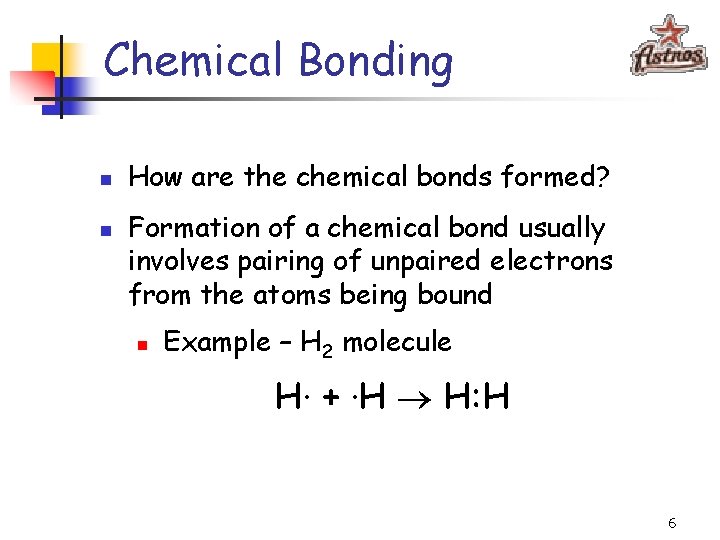
Chemical Bonding n n How are the chemical bonds formed? Formation of a chemical bond usually involves pairing of unpaired electrons from the atoms being bound n Example – H 2 molecule H· + ·H H: H 6
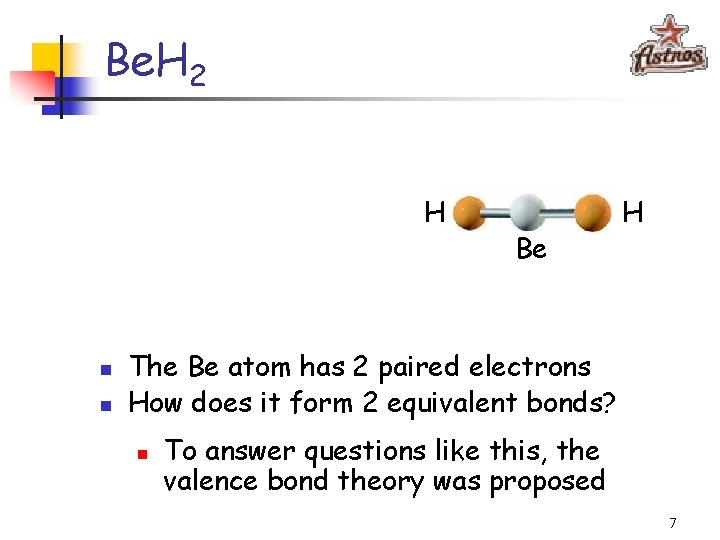
Be. H 2 H n n Be H The Be atom has 2 paired electrons How does it form 2 equivalent bonds? n To answer questions like this, the valence bond theory was proposed 7
Valence Bond Theory n n n When an atom is nearby other atoms, its outer shell orbitals can mix and get modified They form a new set of orbitals that are more appropriate for bonding This process is called hybridization The new orbitals are therefore called hybrid orbitals Hybrid orbitals are arranged in the same way as predicted by VSEPR 8
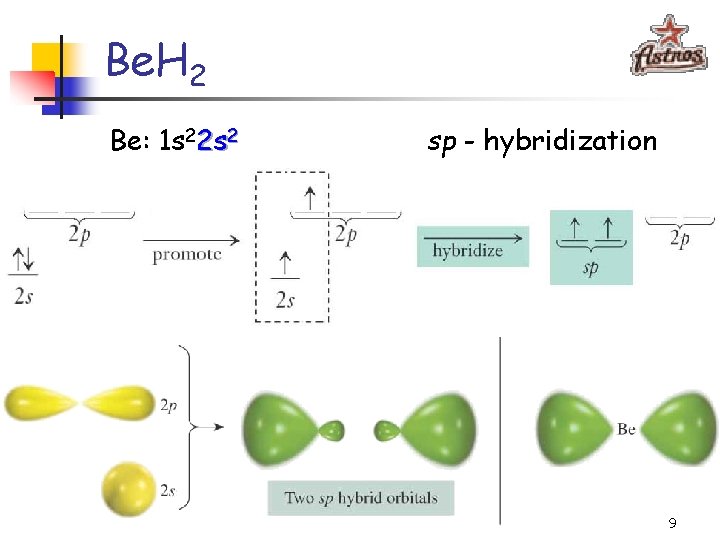
Be. H 2 Be: 1 s 22 s 2 sp - hybridization 9
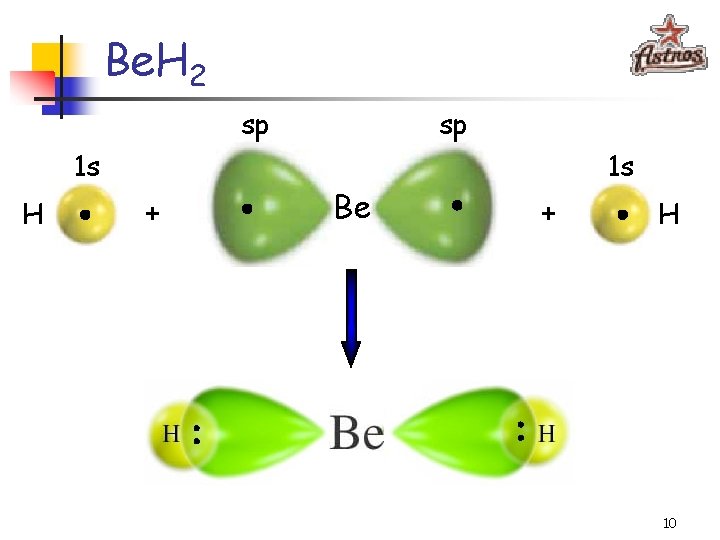
Be. H 2 sp 1 s H + sp Be 1 s + H 10
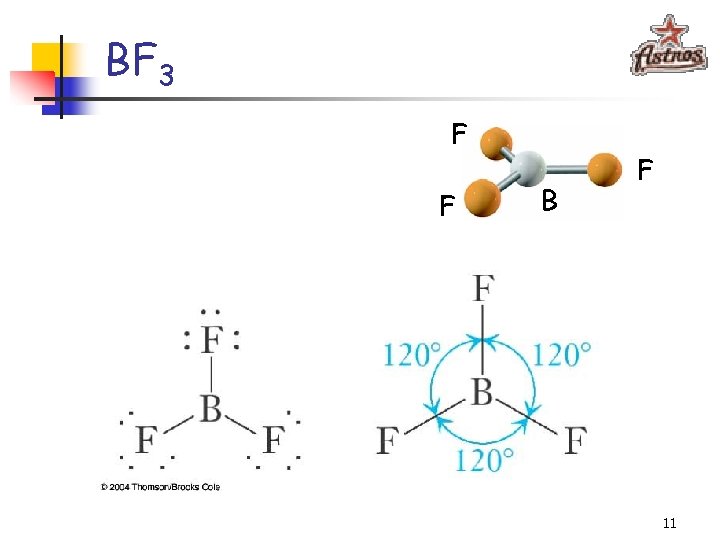
BF 3 F F B F 11
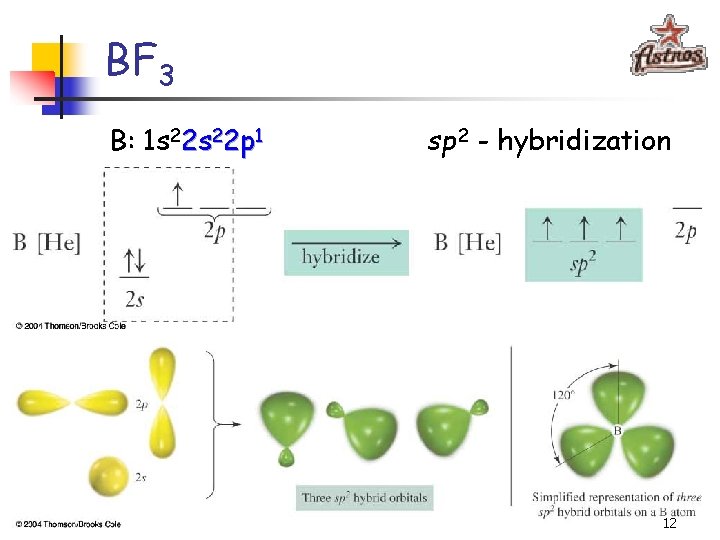
BF 3 B: 1 s 22 p 1 sp 2 - hybridization 12
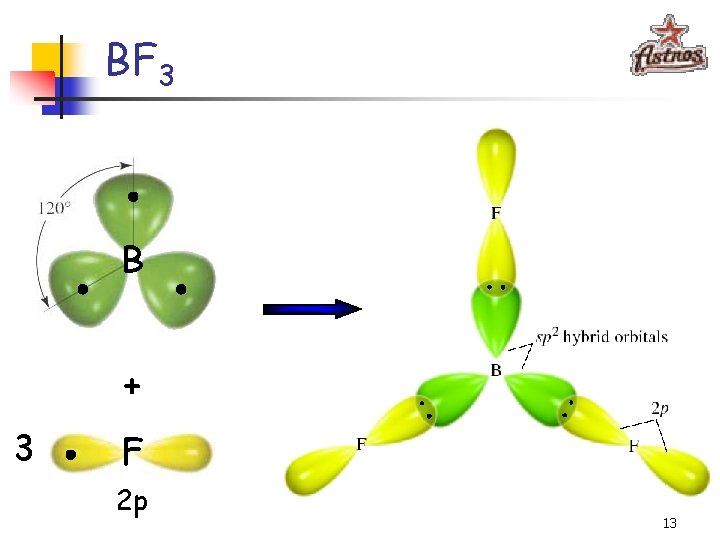
BF 3 B + 3 F 2 p 13
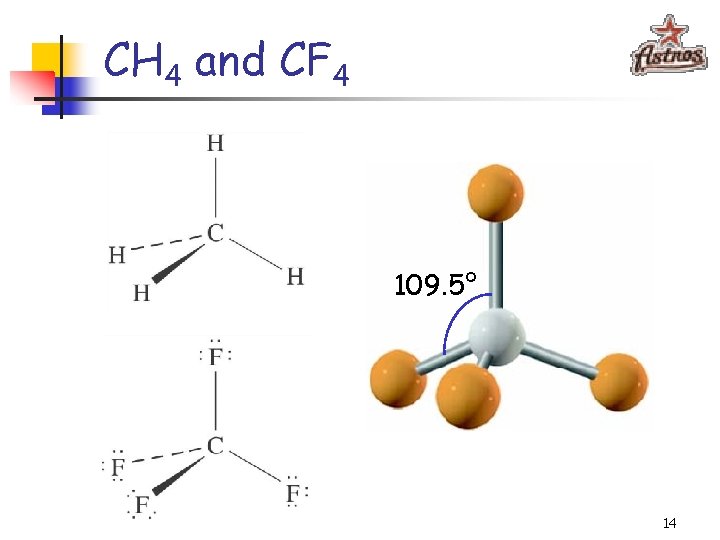
CH 4 and CF 4 109. 5° 14
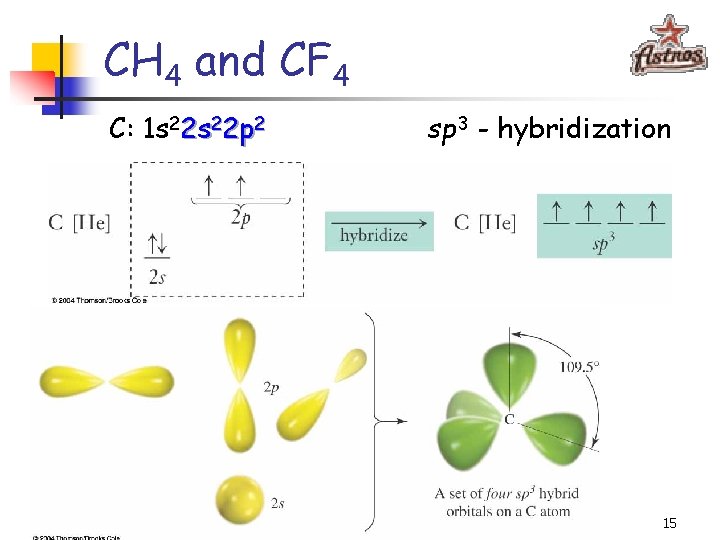
CH 4 and CF 4 C: 1 s 22 p 2 sp 3 - hybridization 15
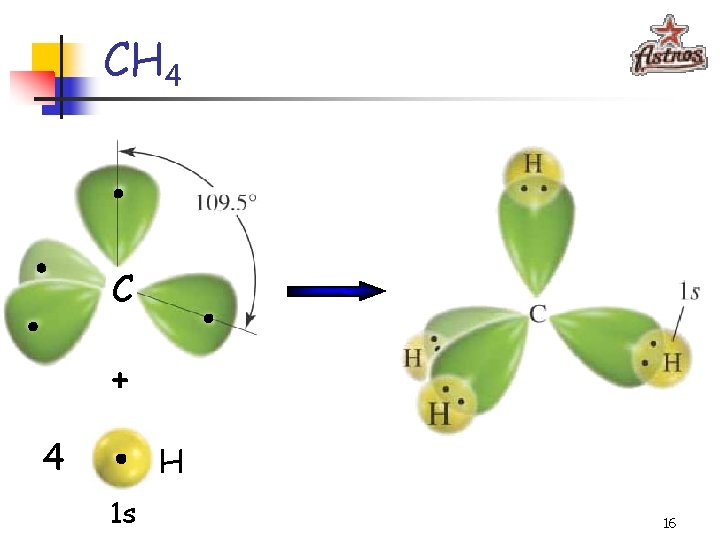
CH 4 C + 4 H 1 s 16
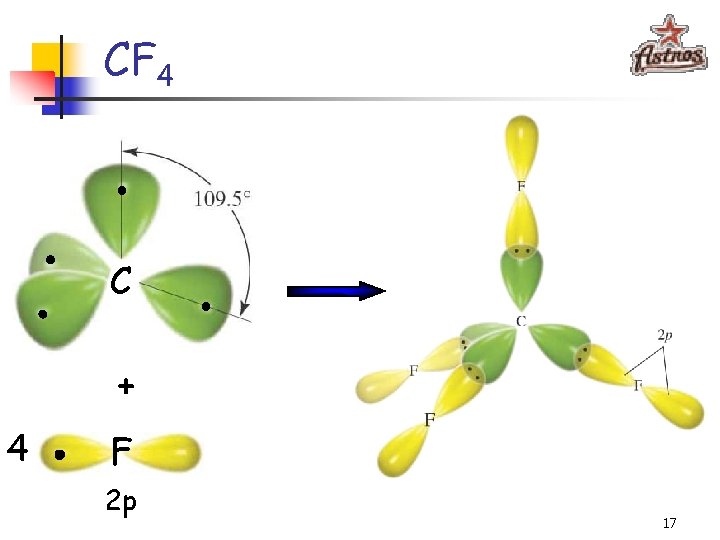
CF 4 C + 4 F 2 p 17
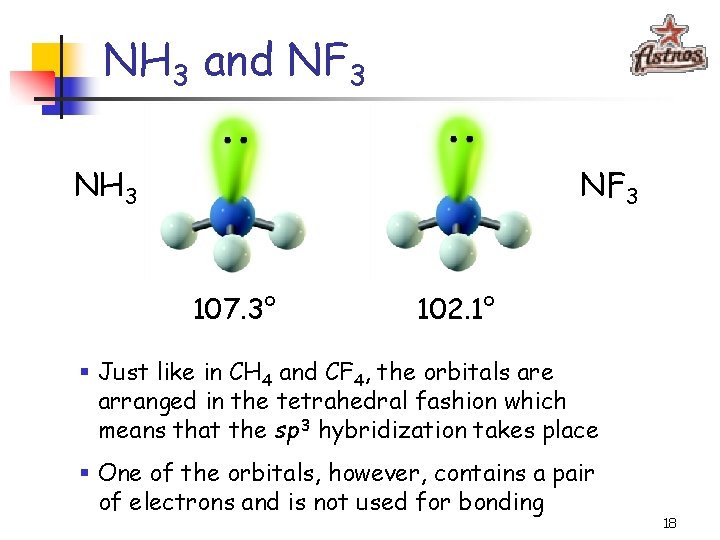
NH 3 and NF 3 NH 3 NF 3 107. 3° 102. 1° § Just like in CH 4 and CF 4, the orbitals are arranged in the tetrahedral fashion which means that the sp 3 hybridization takes place § One of the orbitals, however, contains a pair of electrons and is not used for bonding 18
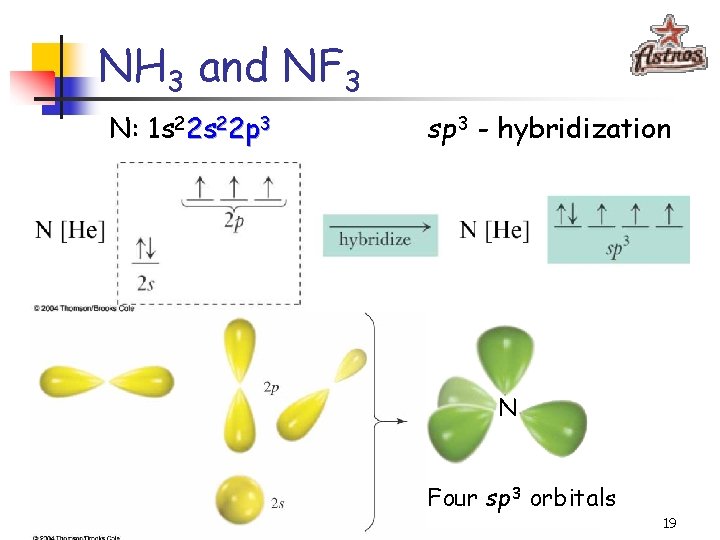
NH 3 and NF 3 N: 1 s 22 p 3 sp 3 - hybridization N Four sp 3 orbitals 19
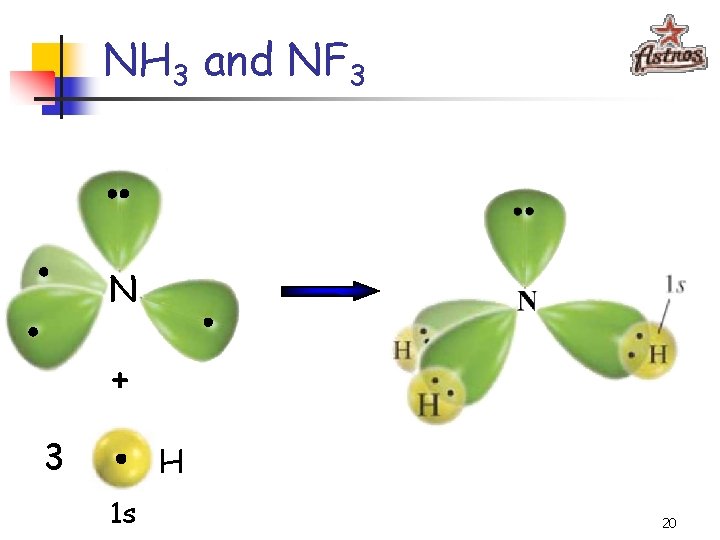
NH 3 and NF 3 N + 3 H 1 s 20
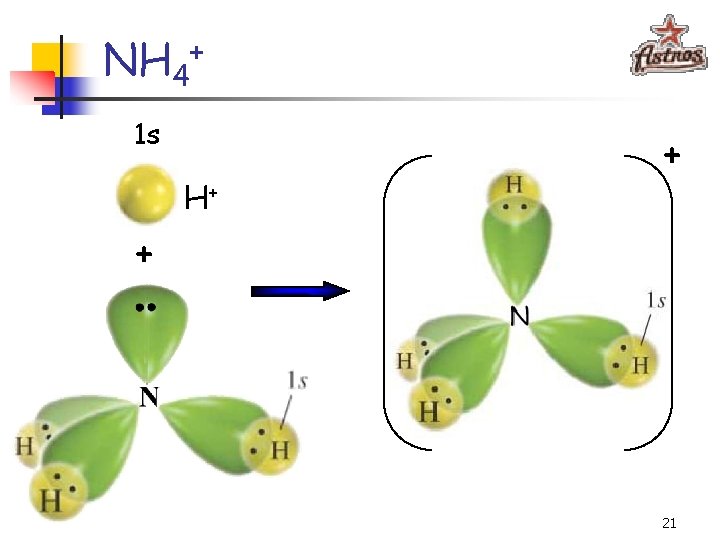
NH 4+ 1 s H+ + + 21
![PF 5 P: [Ne]3 s 23 p 3 sp 3 d - hybridization 22 PF 5 P: [Ne]3 s 23 p 3 sp 3 d - hybridization 22](http://slidetodoc.com/presentation_image_h2/674664d3e939f5bb4d6dc06df1994e1e/image-22.jpg)
PF 5 P: [Ne]3 s 23 p 3 sp 3 d - hybridization 22
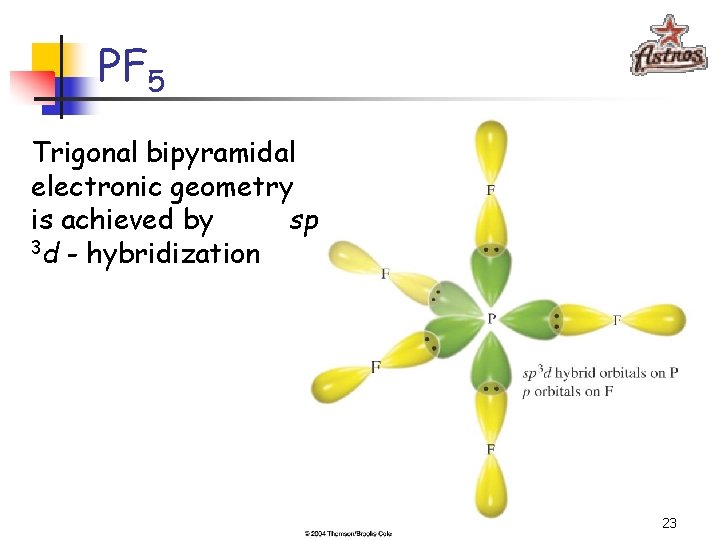
PF 5 Trigonal bipyramidal electronic geometry is achieved by sp 3 d - hybridization 23
![SF 6 S: [Ne]3 s 23 p 4 sp 3 d 2 - hybridization SF 6 S: [Ne]3 s 23 p 4 sp 3 d 2 - hybridization](http://slidetodoc.com/presentation_image_h2/674664d3e939f5bb4d6dc06df1994e1e/image-24.jpg)
SF 6 S: [Ne]3 s 23 p 4 sp 3 d 2 - hybridization 24
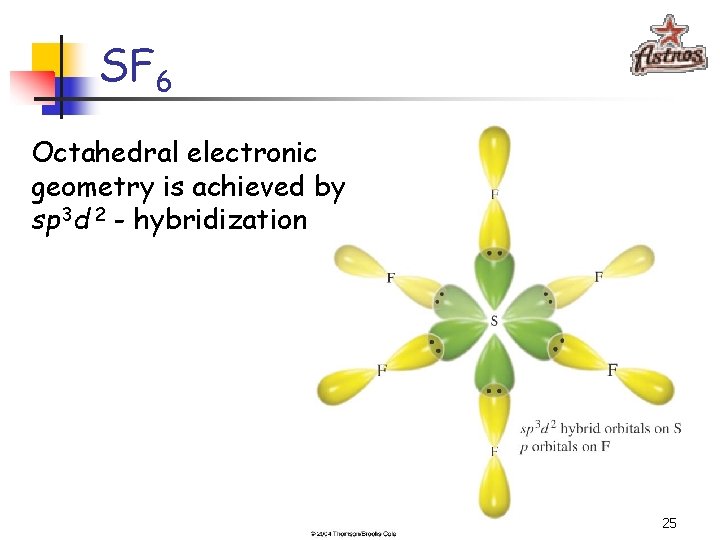
SF 6 Octahedral electronic geometry is achieved by sp 3 d 2 - hybridization 25
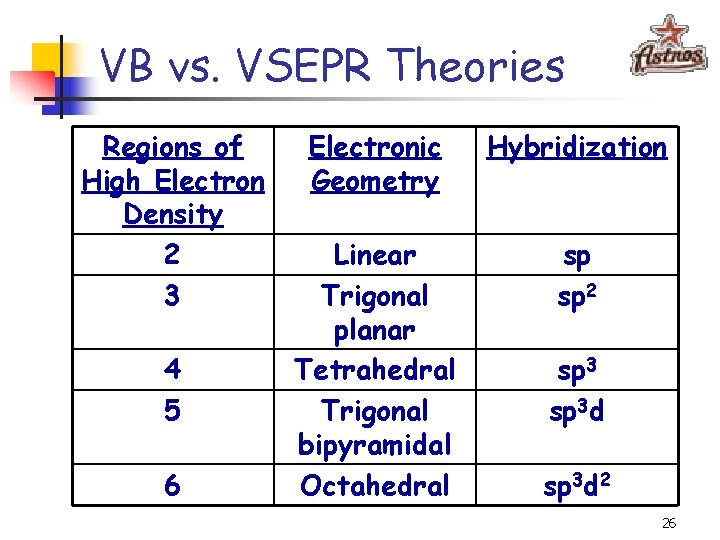
VB vs. VSEPR Theories Regions of High Electron Density 2 3 4 5 6 Electronic Geometry Hybridization Linear Trigonal planar Tetrahedral Trigonal bipyramidal Octahedral sp sp 2 sp 3 d 2 26
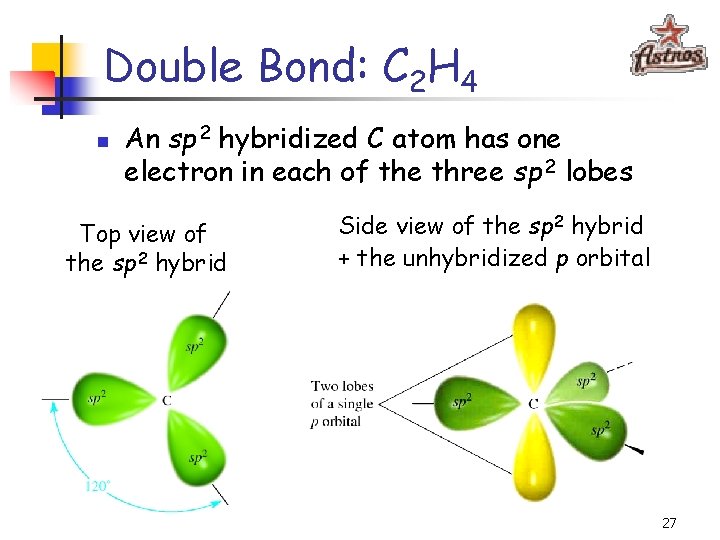
Double Bond: C 2 H 4 n An sp 2 hybridized C atom has one electron in each of the three sp 2 lobes Top view of the sp 2 hybrid Side view of the sp 2 hybrid + the unhybridized p orbital 27
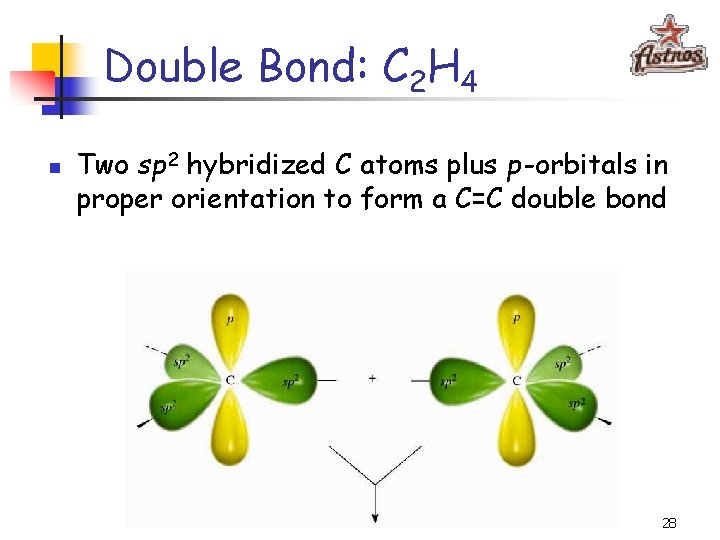
Double Bond: C 2 H 4 n Two sp 2 hybridized C atoms plus p -orbitals in proper orientation to form a C=C double bond 28
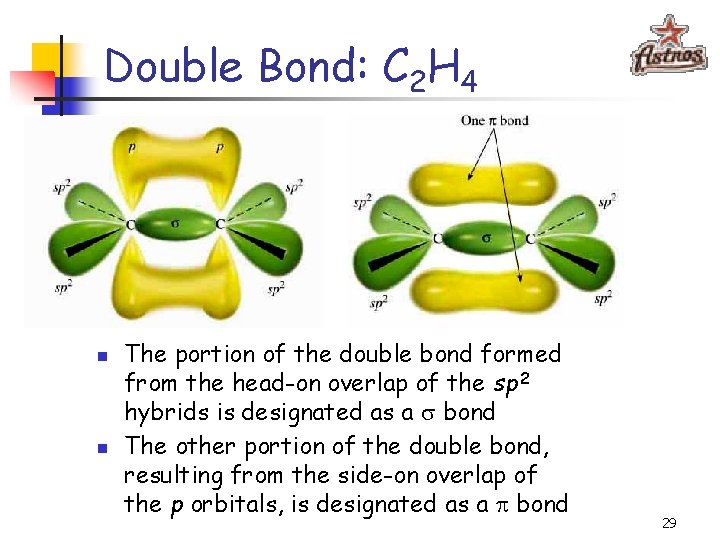
Double Bond: C 2 H 4 n n The portion of the double bond formed from the head-on overlap of the sp 2 hybrids is designated as a bond The other portion of the double bond, resulting from the side-on overlap of the p orbitals, is designated as a p bond 29
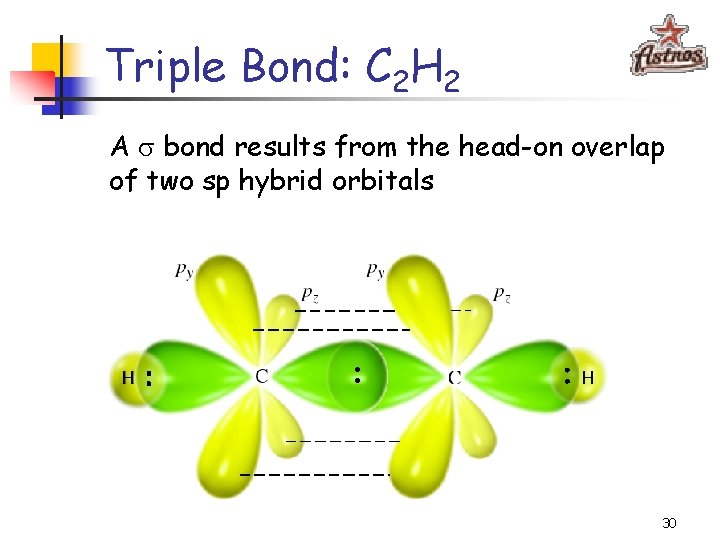
Triple Bond: C 2 H 2 A bond results from the head-on overlap of two sp hybrid orbitals 30
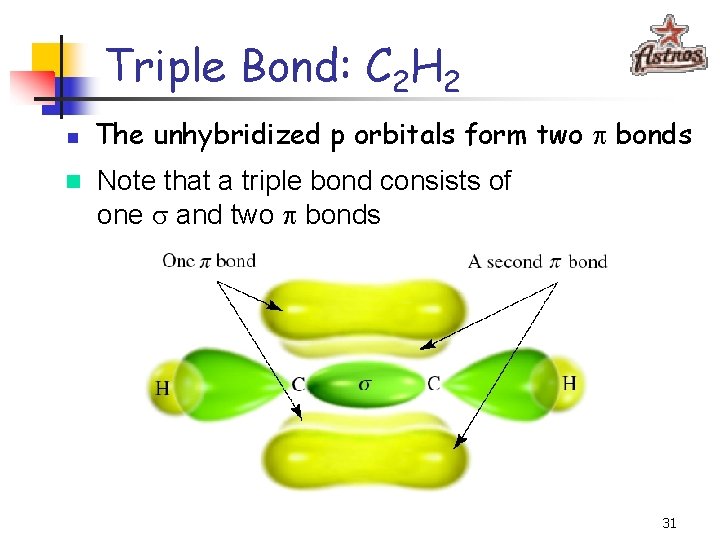
Triple Bond: C 2 H 2 n n The unhybridized p orbitals form two p bonds Note that a triple bond consists of one and two p bonds 31

Assignments & Reminders n Go through the lecture notes n Read Chapter 8 completely n n Homework #5 covers Chapters 7 & 8 and is due by Oct. 31 Monday (10/31) and Tuesday (11/1) – lecture quiz #5 (Chapter 8) 32
- Slides: 32