Fish Health Protocols Chris Mac Williams DVM Principles





















- Slides: 26

Fish Health Protocols Chris Mac. Williams, DVM

Principles of Disinfection: process that reduces or eliminates pathogenic microorganisms Cleaning is the most important step in the cleaning and disinfection process Cleaning process has 5 steps: Dry clean Wet wash Rinse Dry Inspect

Methods of Disinfection Physical – – Moist heat UV light Drying Exposure to sunlight Chemical – – Chlorines (bleach) Iodines (Ovadine) Quaternary ammonium compounds (Diquat, Roccal) Oxidizing agents (Virkon, Ozone)

Chemical Disinfectants Considerations: – Proven efficacy against pathogens of interest: Bacteria, virus, protozoans, fungus, spores – Safety Fish, user, equipment, environment – Affordable – Other: Presence of organic matter Effects on metals/fabrics/rubber

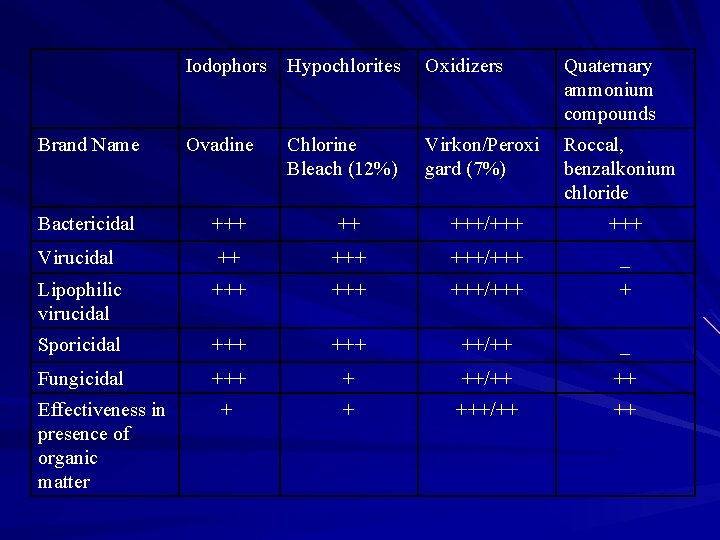
Brand Name Iodophors Hypochlorites Oxidizers Quaternary ammonium compounds Ovadine Chlorine Bleach (12%) Virkon/Peroxi gard (7%) Roccal, benzalkonium chloride Bactericidal +++ ++ +++/+++ Virucidal ++ +++/+++ _ Lipophilic virucidal +++ +++/+++ + Sporicidal +++ ++/++ _ Fungicidal +++ + ++/++ ++ + + +++/++ ++ Effectiveness in presence of organic matter

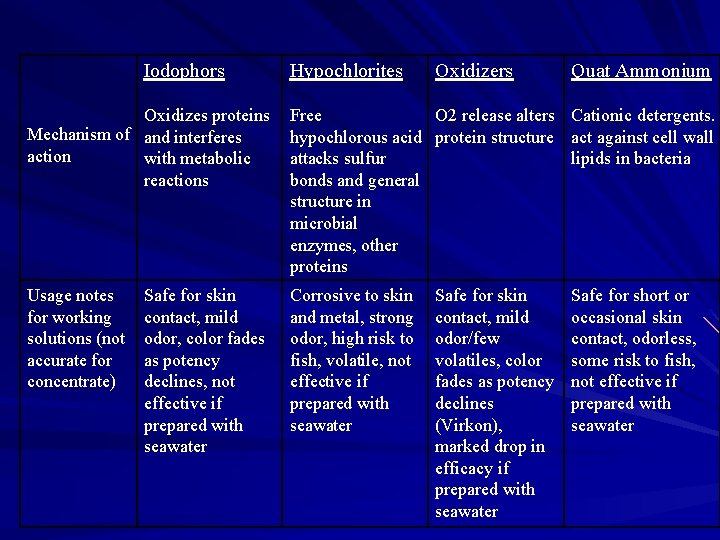
Iodophors Hypochlorites Oxidizers Quat Ammonium Oxidizes proteins Mechanism of and interferes action with metabolic reactions Free O 2 release alters Cationic detergents. hypochlorous acid protein structure act against cell wall attacks sulfur lipids in bacteria bonds and general structure in microbial enzymes, other proteins Usage notes for working solutions (not accurate for concentrate) Corrosive to skin and metal, strong odor, high risk to fish, volatile, not effective if prepared with seawater Safe for skin contact, mild odor, color fades as potency declines, not effective if prepared with seawater Safe for skin contact, mild odor/few volatiles, color fades as potency declines (Virkon), marked drop in efficacy if prepared with seawater Safe for short or occasional skin contact, odorless, some risk to fish, not effective if prepared with seawater


Ovadine Egg Disinfection Why? – Vertically transmitted diseases On the surface of the egg Within an egg – Disinfecting eggs significantly decreases pathogen loads When? – During water hardening – After egg shocking and picking – After eyed eggs are transferred to a site

Ovadine Egg Disinfection How? 100 ppm = 10 mls of Ovadine per litre of water – – – Determine volume of heath tray Add appropriate amount of Ovadine to tray full of water Gently pour rinsed, fertilized eggs into tray preloaded with Ovadine solution – After 10 minutes without disturbance, restore water flow

Ovadine Egg Disinfection 1 volume of eggs : 10 volumes of disinfectant solution Solution colour indicates efficacy Brown = active Yellow = inactive and should be refreshed Make a fresh Ovadine solution for each lot of eggs

Ovadine Egg Disinfection Spent solution disposal – Dilution is standard, combined with hatchery effluent, or can be disposed to ground Neutralize: Sodium thiosulfate 0. 78 X grams of iodine x 2 (safety factor) or 0. 15 gm per litre of 100 ppm solution Water should be colourless before discharge to ground

Managing Egg Fungal Infections Egg picking – By hand – Mechanical pickers Chemical treatments – Formalin (Parasite-STM) – Hydrogen Peroxide – Salt – Bronophol (EDR) – Malachite Green

Egg Picking Dead eggs are food for fungus After shocking eyed eggs – observe eggs for mortalities

remove dead eggs promptly

Early – remove affected

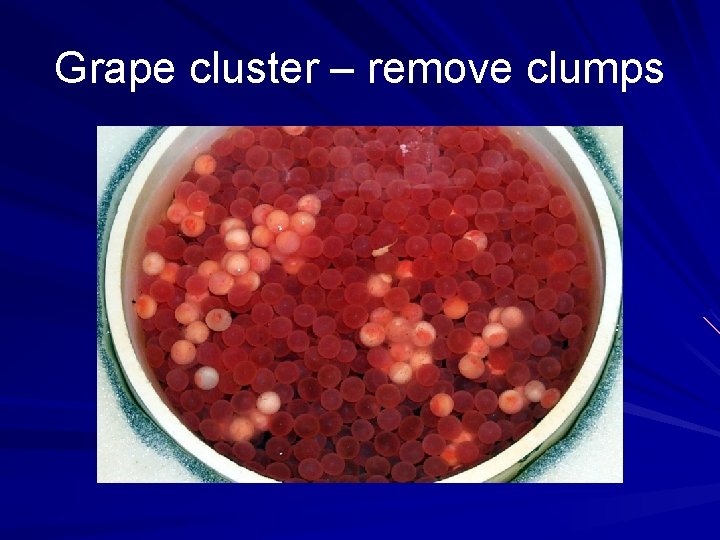
Grape cluster – remove clumps

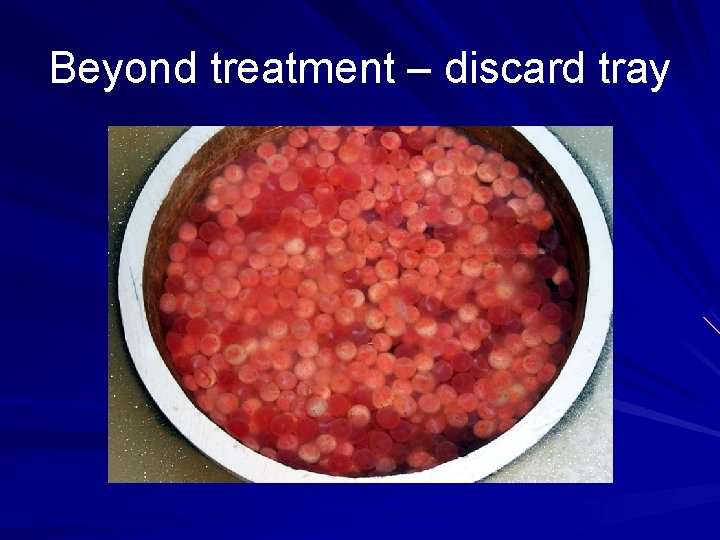
Beyond treatment – discard tray

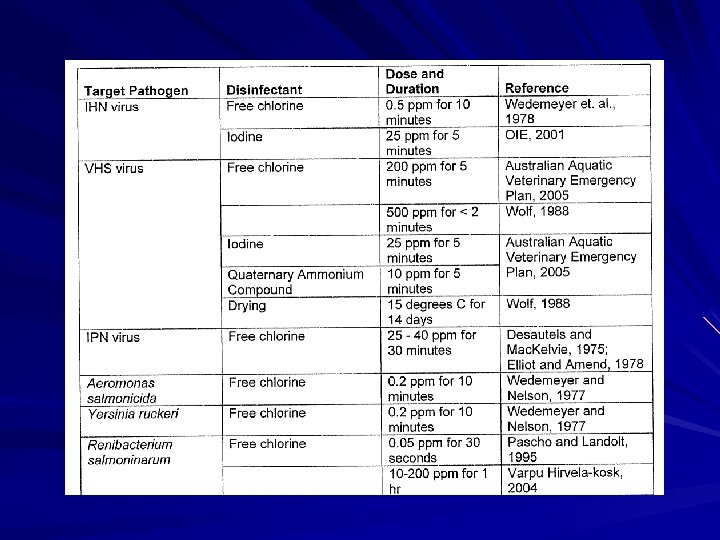
Formalin Egg Disinfection 1670 ppm for 15 – 20 minutes Static bath till last few weeks till hatch – Then keep water flow >11 lpm Hanging IV bags or chicken feeders

Hydrogen peroxide (Perox-Aid) 500 ppm for 30 – 35 minutes daily Comparatively environmentally benign Will not work on established fungal infections – only preventative

Others Salt – Robertson Creek every 2 – 3 days Bronophol – no benefits vs approved Malachite Green – absolutely not!

Sample Shipping to a Diagnostic Facility Mortalities are unexpected Clinical signs are suggestive of a disease of concern (eg. popeye and/or swollen abdomens at a facility with a history of recurrent BKD infection) Daily mortality rate exceeds 0. 5% of the population

Selecting the samples Moribund fish preferred There may be a need to randomly sample apparently healthy fish from the population Ask Fish Path Lab staff re: sample type, numbers and shipping info

Before shipping Collect fish history: – – – population size clinical signs mortality and morbidity rate diet and feed consumption water quality conditions records of recent stressful events (e. g. low water event, marking) – vaccination status – disease and treatment history Fill out a submission form

Shipping Live Fish Line a cooler with ice paks or double bagged ice Cover ice with newspaper Live fish are added to heavy duty plastic bag filled ¼ to 1/3 full of aerated ambient water Oxygen is pumped into the bag to refill it. The bag is securely closed off using elastic bands or tape. Double bag and placed in the Styrofoam cooler. Each bag is clearly marked with information identifying contents. The remaining space in the cooler is filled with cubed ice to keep the fish and water cool. The lid is placed on the Styrofoam cooler and securely fastened with duct tape to prevent accidental spillage.

Shipping Include a pathology laboratory submission form or an accompanying letter with more detail Include copy of mortality records if available Clearly mark sample bags The container is addressed to the laboratory at PBS and is clearly labeled with information as to originating site. Contact lab with estimated time of sample arrival

Shipping fresh dead fish Fresh morts (red gills, firm flesh) should be placed in labeled, sealed double plastic bags without water. Ship dead fish in a container on ice as described above for live fish. Fish should not come in contact with the ice or freezer packs.