First Year Experience Research Lab Safety 1 PPE

First Year Experience Research Lab Safety

1. PPE Personal Protective Equipment: What must be worn when you work in the laboratory. Eye Protection Lab Coat Long Pants Closed Toed Shoes and Socks – no exposed skin around feet Lab gloves – when required 2

Eye Protection • Contact lenses are OK as long as glasses/goggles are worn • Prescription glasses – you must wear goggles over them • Safety goggles are provided in organic labs in UV irradiating cabinets • Eye wash stations are present in all labs 3

Clothing and Foot Protection • Clothing must cover all exposed skin including legs/ankles • Socks as required PPE • Stockings or leggings do not provide good coverage • Sandals, flip-flops, Crocs, open-toe and open-top (i. e. ballet flat) shoes and canvas shoes (i. e. Toms) are not appropriate. These are not going to protect your feet if you drop a piece of glass with a liquid chemical reagent in it. 4

Be smart about the shoes you elect to wear to lab This person has on pants and closed toed-shoes but this would not be allowed in lab due to the exposed skin This person added socks, so this option covers your skin but only offers minimal protection This option looks better, but imagine chemicals being spilled into the top of these boots Your best options are sturdy leather footwear that covers the entire top of the foot or a sturdy running shoe.

Use of Gloves Remove gloves before handling objects such as doorknobs, telephones, pens, computer keyboards, p. H meter or other electronic buttons, or phones while in lab. It might be convenient to have one gloved hand one ungloved hand to do procedures where these kinds of things are used. • Throw away gloves anytime you take them off. • You should expect to use several pairs of gloves in any given lab period. 6

Glove Recycling Contaminated Gloves with visible signs of chemical exposure or those used with hazardous substances should be collected in solid waste or biohazard containers. Uncontaminated Gloves that have no visible sign of chemical exposure or residue can be placed in the glove recycling container. If there’s any questions or the boxes need attention, please contact Dr. Katherine Mullaugh. Email: mullaughkm@cofc. edu Phone: 843. 953. 6587 7

Eyewash / Safety Shower The eyewash is on the left. Pull the handle and a fountain of water will appear that you can use to bathe your eyes. The safety shower is on the right. Pull the handle and water will start spraying from the shower head on the ceiling. There’s no drain in the floor – we only do this in emergencies, because a flood of water will have to be cleaned up. 8

Eye Wash 9

Safety Shower 10

Using the Fume Hoods properly This window/bar is called the sash. If this is not saying NORMAL, then the hood is not protecting you. Keeping the sash and sliding panels in proper position keeps this NORMAL, otherwise the alarm goes off. If the alarm goes off, you need to reposition things to the correct positions, then press the “mute” button to reset the controller. The sash should never be raised above the green “operation” level when you 11 are working in the hood.
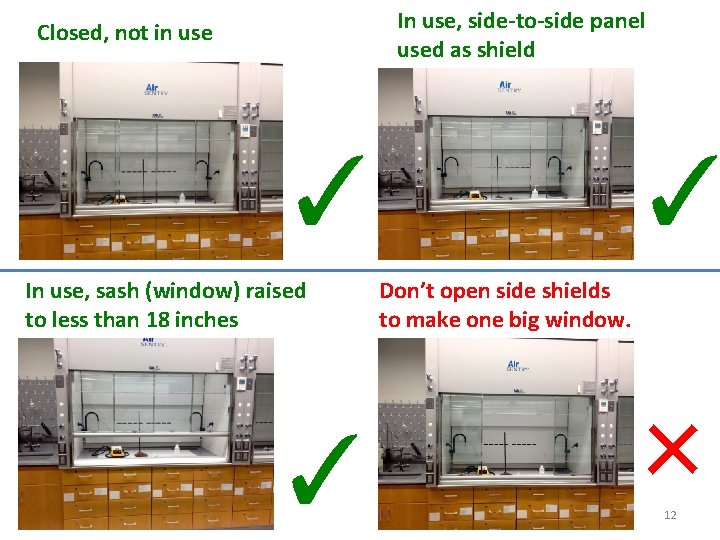
In use, side-to-side panel used as shield Closed, not in use ✓ In use, sash (window) raised to less than 18 inches ✓ ✓ Don’t open side shields to make one big window. × 12

• When using a laboratory hood, Check that the airflow is in the normal range on the digital display • Turn on the hood light • Set the equipment and chemicals back at least 6 inches. • Never lean in and/or put your head in the hood when you are working. This is worse than doing the experiment with no hood at all. • It’s a good idea to put liquid reagent containers in trays to catch all spills and drips 13

Fire Alarms – know the location of one close to your lab 14

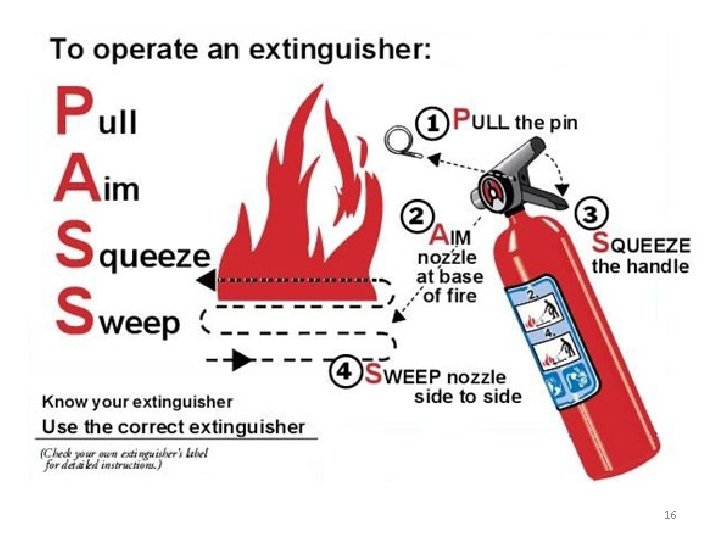
Fire Extinguishers – we have several in the labs and in the hallways. 15
16

Types of Fire Extinguishers This is a special fire extinguisher for combustible metal fires. It is a type D fire extinguisher. You won’t need to use this unless you work in a research lab with combustible metals. Most of our fire extinguishers are ABC. It contains a dry powder to put out the kinds of fires we might encounter in the chemistry labs where we have class. 17

Student Reaction in a Fire Although we want you to be informed on the operation of a fire extinguisher, we do not expect you to use it. If a fire is ignited in your area, the proper STUDENT response is to: 1) Notify everyone in the room 2) If possible shutdown any reaction in progress by removing heat/energy source 3) Proceed to the nearest exit and pull the nearest fire alarm 4) Evacuate the building 5) Assemble in front of the library or in the YWCA parking lot for a positive headcount 18

Keep your lab area clean. × Throw away used paper towels and used gloves, immediately. × Don’t block the floor in front of the eyewash/shower station. × Don’t leave cords dangling because someone will trip over them. × Don’t leave things in the floor because someone will trip over it. 19

Injury procedure • First Aid kits are available in the lab with band aids and other items for treating small cuts and burns. • Campus public safety can be reached at 35609 for non-emergencies. • If it is a serious injury, call 911 for emergencies. • The Live. Safe app can also be used to report emergencies and non-emergencies. 20

Centrifuge Safety

Rotor Safety • Do not run rotors above their rated speed • Inspect rotor for imperfections and signs of wear that can eventually lead to catastrophic failure • Do not drop rotor • Rinse the rotor after every use • Avoid using abrasive brushes for cleaning • If you suspect rotor has been damaged, do not use it • Do not use a rotor that is not compatible with your model centrifuge • Use tubes and adapters that are rated for use in the rotor being used Disposable tubes Fixed angle rotor Swinging Bucket rotor Need adaptors
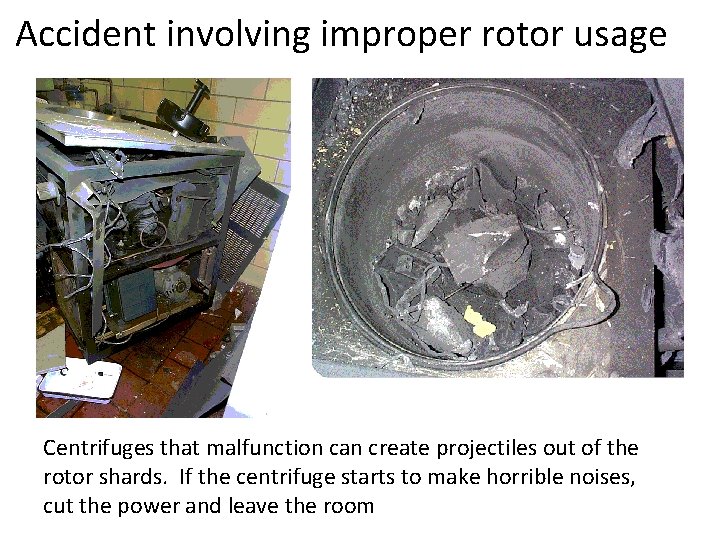
Accident involving improper rotor usage Centrifuges that malfunction can create projectiles out of the rotor shards. If the centrifuge starts to make horrible noises, cut the power and leave the room
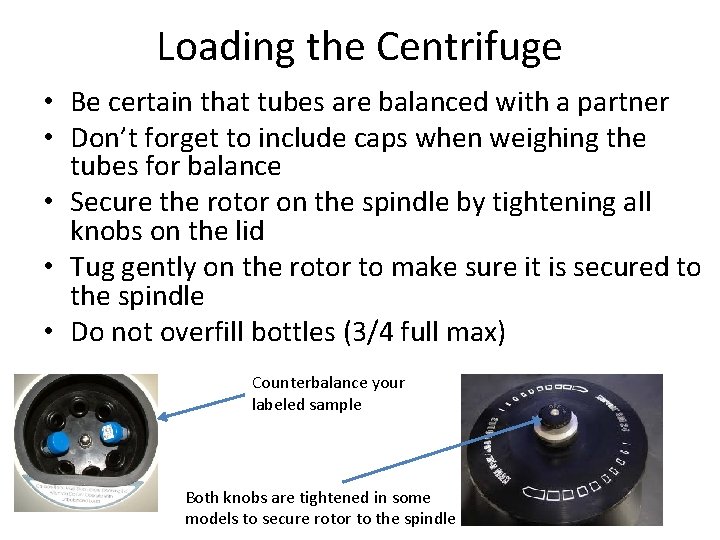
Loading the Centrifuge • Be certain that tubes are balanced with a partner • Don’t forget to include caps when weighing the tubes for balance • Secure the rotor on the spindle by tightening all knobs on the lid • Tug gently on the rotor to make sure it is secured to the spindle • Do not overfill bottles (3/4 full max) Counterbalance your labeled sample Both knobs are tightened in some models to secure rotor to the spindle

Unloading Centrifuge • Take precautions if biohazards or other hazardous material is used as aerosols can form during vacuum cycles • Clean the chamber from condensation and any spills • Never try to open the centrifuge door before the rotor is done spinning • Never reach a hand or anything else into the chamber when rotor is spinning • Note: it is sometimes difficult to look at a spinning rotor and determine if it is spinning

Centrifuge Safety Overview • http: //www. youtube. com/watch? v=q_0 ph. A 0 34 n 0 Note: A modern centrifuge will have low tolerance for mismatched tubes and will shut itself off if tubes are not balanced Also, most modern centrifuges will not allow the door to unlock while the rotor is still in motion.

Autoclave Safety 27

What is an Autoclave? An autoclave is a specialized piece of equipment designed to deliver heat under pressure to a chamber, with the goal of decontaminating or sterilizing the contents of the chamber.

Personal Protective Equipment • Autoclaves utilize steam, heat and pressure and therefore the risk of personal injury through scalding, burns and exploding glassware is great. • Personal protective equipment is absolutely required. 1) Safety Glasses 2) Lab Coat 3) Long pants 4) Closed Shoes 5) Socks 6) Long thermal gloves 7) Face shield recommended

What can be autoclaved? • • Cultures and stocks of infectious material Culture dishes Tips, pipettes, gloves, paper towels, aluminum foil Centrifuge bottles Glassware -- all caps must be loosened Media and other aqueous solutions Contaminated solid items

What CANNOT be autoclaved? • • • Solvents or volatiles Chlorinated compounds (HCl, bleach) Corrosives Radioactive material Some plastics
Cycle Differences • Fluids must be autoclaved under a “liquid” setting • Items such as pipette tips, test tubes, and centrifuge bottles are run under “dry” or “gravity” setting • The difference in settings is how the cycle is vented • Liquids must depressurize slowly and dry cycles conclude with a vacuum step to draw off condensation

Loading and Unloading the Autoclave • All screw caps must be loosened to prevent pressure changes in the glassware that can cause the container to burst • All items should be placed in an autoclave tray to prevent scald burns in the event of a spill • Return autoclave trays promptly so that other users do not skip using a tray because they can’t find one • Don’t skip using a tray • Do not remove liquid that is still boiling • If possible, allow glassware to cool before removing Loosen cap by several threads

Door Safety • Never try to open a door that is under pressure • Never try to speed up the venting process by tampering with the door, by turning on and off the machine, etc. Venting takes time. • Know where the pressure gauges are for the instrument you are using • If possible, vent door slowly

Autoclaving Waste This bag is too full • Contaminated pipette tips and solid waste should be sterilized prior to disposal • Collect waste in a special autoclave-safe biohazards bag • Place bag in secondary container • Vent the bag by opening • Do not overfill bag • After removal place entire bag in a new trash bag so that “biohazard” signs are no longer showing • Sterilized waste can go into the normal trash • Autoclave tape can be used to verify heat delivery but it does not guarantee proper sterilization

Container Choice • Pyrex glass, metal, polypropylene (PP) plastic and polycarbonate (PC) plastic are best choices • Polyethylene (PE), polystyrene (PS), and high density polyethylene (HDPE) will often melt and make a mess

Autoclaving Tips • Add a 2 cm depth of water to trays with glassware; the water helps eliminate air pockets between the tray and the glass and helps prevent glass from breaking • Do not fill liquid past 75% volume • Separate items to increase steam penetration • Increase cycle time for large volumes of liquid • Temperature must be maintained at 121°C for at least 30 minutes for liquid loads

Maintenance • Report any irregularities to your supervisor • Do not operate if there is a steam outage • Failed runs should be reported and logged

Other Biochemistry Safety Concerns

Toxic and Health Hazardous Chemicals • Categories: – – – – Irritants Sensitizers Carcinogens, Acute toxicity, fatality Corrosives reproductive toxins, target organ damage Carcinogens Target Organ Effects Reproductive Health Toxins Acute Toxins Irritants, sensitizers, Corrosives acutely toxic Physical Health Hazards • Common routes of exposure in the lab are inhalation and skin absorption, while ingestion is less common.
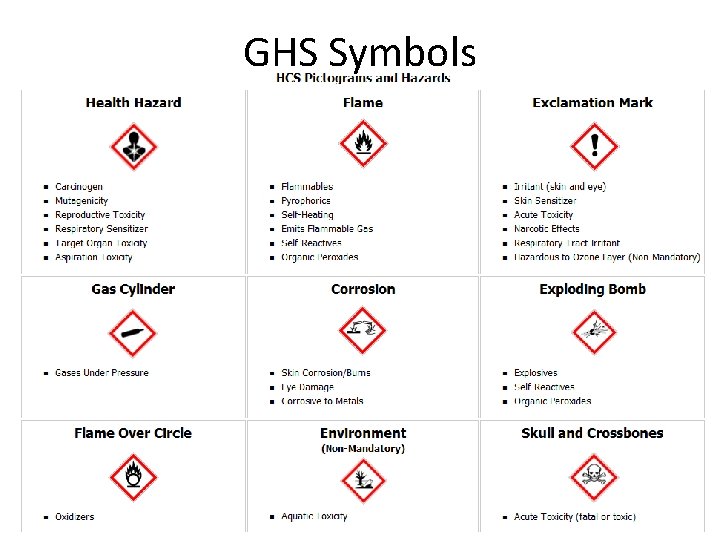
GHS Symbols

Biological Hazards • Biological hazards are potential sources of infectious agents that could be harmful to human health. – Bacterial, fungal, parasitic, viral, and prion agents. – Sources may include animals, tissues, cells, blood, and nucleic acid samples, including recombinant DNA.
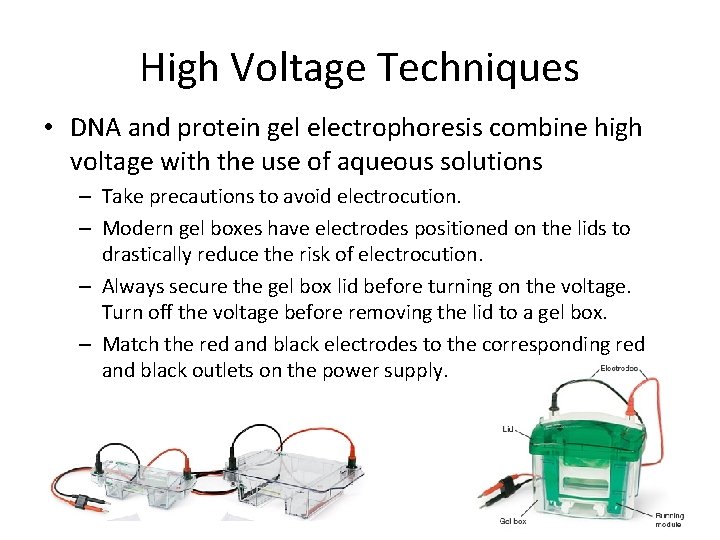
High Voltage Techniques • DNA and protein gel electrophoresis combine high voltage with the use of aqueous solutions – Take precautions to avoid electrocution. – Modern gel boxes have electrodes positioned on the lids to drastically reduce the risk of electrocution. – Always secure the gel box lid before turning on the voltage. Turn off the voltage before removing the lid to a gel box. – Match the red and black electrodes to the corresponding red and black outlets on the power supply.
Ultracold (-80°C) Freezer Use • Many biological samples and chemicals need to be preserved at temperatures below room temperature – Always consult the label: 4°C (refrigerator), -20°C (conventional freezer), -80°C (ultracold freezer), -196°C (liquid nitrogen) – Cold storage can slow cell death rate, preserve enzyme activity, inhibit contaminating bacterial growth, and prevent degradation. – Use insulated gloves to handle ultracold materials. Keep the -80° – Handle glass dewars with caution – danger of freezer closed! exploding glass if they are knocked over and broken.

Liquid Nitrogen Safety • Liquid nitrogen (LN 2) is commonly used to rapidly freeze proteins and bacteria • LN 2 rapidly evaporates and can displace air in enclosed spaces causing suffocation • LN 2 can cause death of human tissue from extreme cold • Minor contact can cause “burns” • Evacuated glass dewars can sometimes burst unexpectedly • LN 2 can condense liquid oxygen

Liquid Nitrogen DON’T’s DON’T use in confined space DON’T freeze items in centrifuge tubes with snap caps DON’T transport LN 2 in a closed automobile DON’T transport LN 2 in a passenger elevator DON’T allow a storage dewar to tip over DON’T leave cold fingers on a vacuum line in LN 2 overnight • DON’T use without PPE! • • • NO!

Liquid Nitrogen Do’s YES! • DO use or dispense LN 2 only in well ventilated areas • DO ensure glass dewars are taped or wrapped • DO use approved containers only such as a dewar or NO! threaded cryovials for storage • DO make sure any vessel with LN 2 is VENTED • DO secure storage dewars against spilling • DO use appropriate PPE which includes: • Face shield (or minimally goggles) • Long thermal gloves • Apron or lab coat YES! • Closed toed Shoes • Socks • Long pants

Report any concerns • If you have any safety concerns about the lab you are working in or the people working around you, you can contact: – Your lab instructor – Dr. Marcello Forconi– Head of the departmental safety committee – Dr. Pamela Riggs-Gelasco – Department Chair for Chemistry and Biochemistry – Dr. Jim Deavor, Associate Dean of the School of Science and Mathematics.
- Slides: 48