First stageDepartment of Environmental Health Environmental pollution Lec















- Slides: 23

First stage/Department of Environmental Health Environmental pollution Lec 2 Air pollution Mohammed Al-Jawasim

The atmosphere as a resource • The atmosphere is a gaseous envelop surrounding earth. • The atmosphere are comprised by four major gases which are nitrogen 78. 08%, oxygen 20. 95%, argon 0. 93% and carbon dioxide 0. 04%. The atmosphere contains water vapor and air pollutants. • The atmosphere blocks the earth surface from receiving much of the uv coming from the sun.

The atmosphere as a resource cont. • Oxygen and carbon dioxide are the most important gases for human and living organisms. • During photosynthesis, plants, algae and certain types of bacteria use carbon dioxide to synthesize sugar and organic molecules and produce oxygen in this process. • During cellular respiration, most organism use oxygen to break down food molecules and supply themselves with chemical energy, and this process produces carbon dioxide.

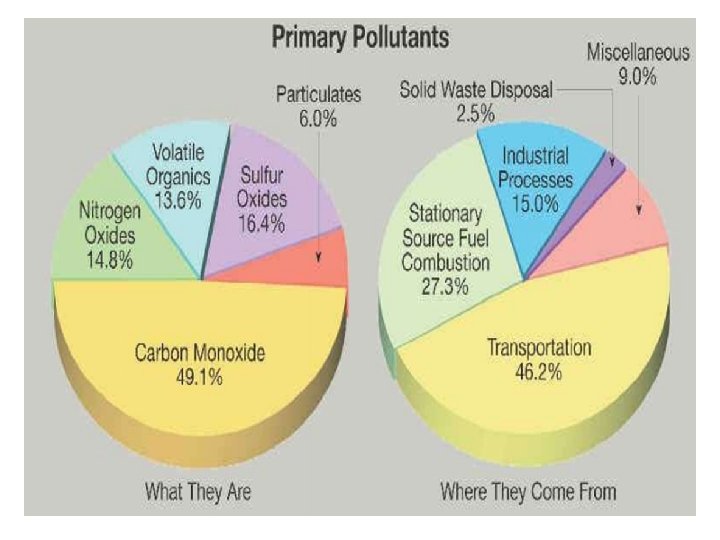
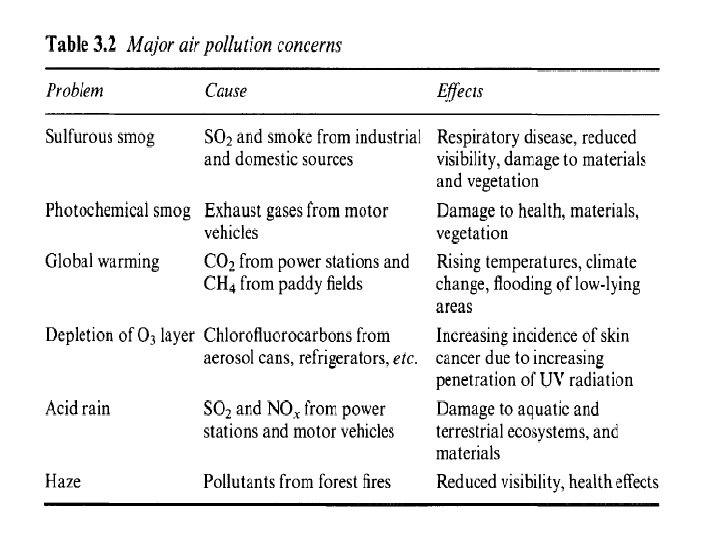
Types and sources of air pollution • Air pollution: various chemical added to the atmosphere by natural events or human activities in high enough concentrations to be harmful. • Air pollution consists of gases liquids or solids present in the atmosphere in high enough levels to harm human, other organisms or materials. • Air pollution can be caused by natural and human activities • Many different air pollutants exist such as particulates matter, nitrogen oxides, sulfur oxides, carbon oxides, hydrocarbons, ozone and air toxics.

Air pollutants types • Air pollutants are classified into two different types, primary air pollutants and secondary air pollutants. • Primary air pollutants are harmful substances such as soot or carbon monoxide that is emitted directly into the atmosphere 1. 2. 3. 4. 5. Carbon oxides, nitrogen oxides, sulfur dioxide, particles matter and hydrocarbons major examples of primary air pollutants.


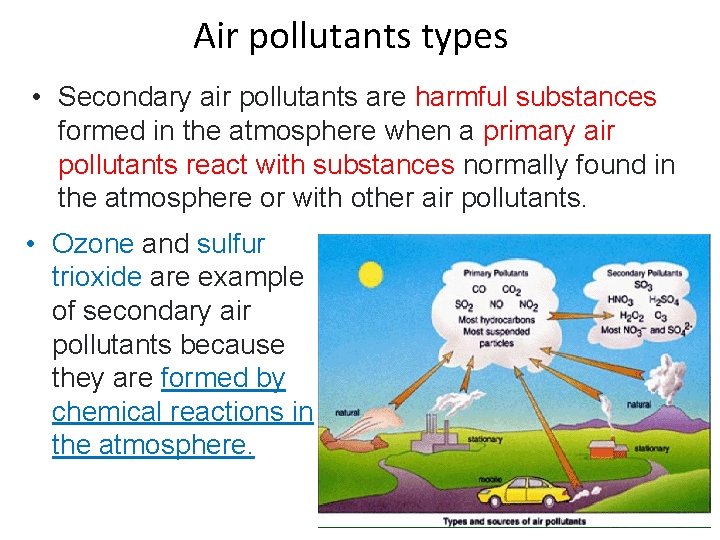
Air pollutants types • Secondary air pollutants are harmful substances formed in the atmosphere when a primary air pollutants react with substances normally found in the atmosphere or with other air pollutants. • Ozone and sulfur trioxide are example of secondary air pollutants because they are formed by chemical reactions in the atmosphere.

Major air pollutants • 1 -Carbon monoxide (CO) • CO is a colorless, odorless, flammable gas, a product of incomplete combustion produced when a carbon-containing material is burned. • Only under ideal conditions, with an excess of oxygen and optimal burning conditions, is carbon completely oxidized to carbon dioxide (CO 2). • Carbon monoxide (CO) is pervasive. CO accounts for ~ 50% of air pollution by weight nationwide and worldwide.

Why CO is of concern? 1. CO causes up to 11% of hospital admissions for congestive heart failure in older people. 2. The brain too demands a steady oxygen concentration for optimum functioning, so a lowered level of oxygen cause headache, dizziness, fatigue, and drowsiness. 3. Higher doses of CO, such as found in enclosed spaces, may lead to coma and death.

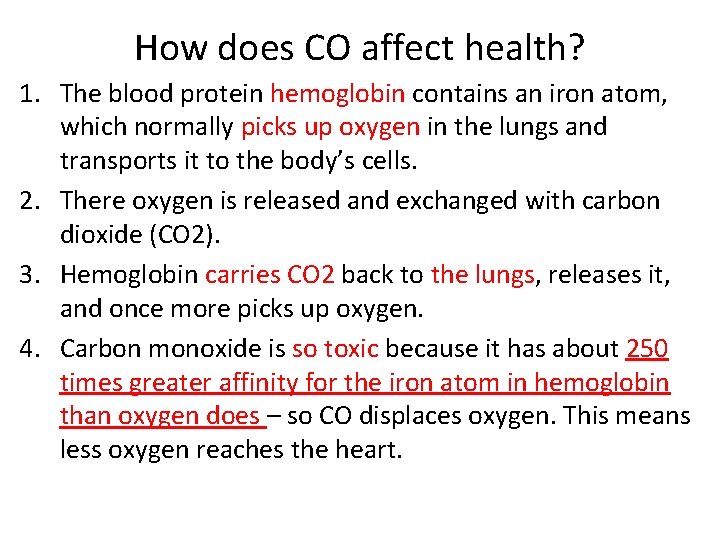
How does CO affect health? 1. The blood protein hemoglobin contains an iron atom, which normally picks up oxygen in the lungs and transports it to the body’s cells. 2. There oxygen is released and exchanged with carbon dioxide (CO 2). 3. Hemoglobin carries CO 2 back to the lungs, releases it, and once more picks up oxygen. 4. Carbon monoxide is so toxic because it has about 250 times greater affinity for the iron atom in hemoglobin than oxygen does – so CO displaces oxygen. This means less oxygen reaches the heart.

2 -Ozone (O 3) • Many of us know the odor of ozone from hot summer days in the city, lightning storms, or improperly maintained electrical equipment such as a photocopier. • O 3 is a summer pollutant in photochemical smog • Most ground-level O 3 comes from human activities. • Ozone is found naturally even in areas remote from human activity, but natural levels are low, 0. 02– 0. 05 ppm • Ground level O 3 is the same O 3 found in the stratosphere. • However, ground level (tropospheric) O 3 is a pollutant whereas in the stratosphere ozone performs a major positive function.

Why O 3 is of concern? • The EPA considers ozone the most serious and persistent air quality problem in the United States, describing O 3 as the “most. . . Intractable air pollutant in urban air. ” • O 3 is often present at levels known to have deleterious effects on health and natural life.

Health impacts 1. Acute health effects of ozone exposure are irritation of eyes, nose, throat, and lungs and a decreased ability of the lungs to function optimally. 2. At 0. 2 ppm young adults develop inflammation of the bronchial tubes and tissue deep within the lungs. 3. Even at the legal limit of 0. 075 ppm, O 3 adversely affects some people, including some healthy individuals 4. People with asthma or bronchitis, especially children, are highly susceptible to O 3, which can also increase susceptibility to infection. 5. Chronic O 3 exposure can permanently damage lungs.

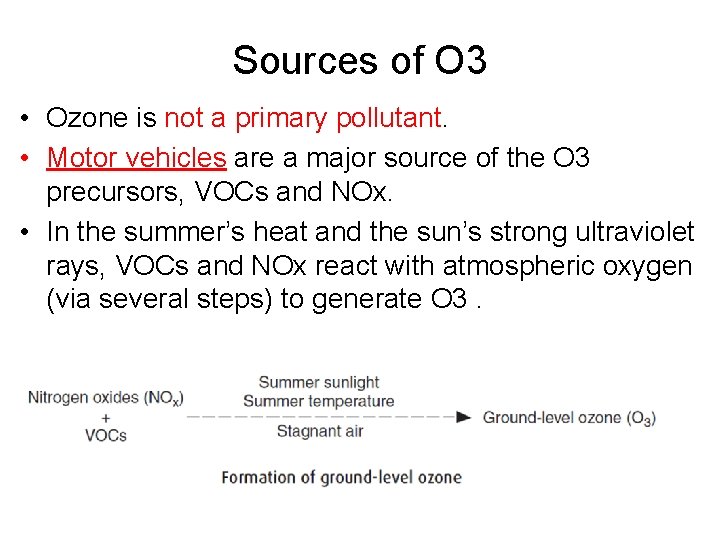
Sources of O 3 • Ozone is not a primary pollutant. • Motor vehicles are a major source of the O 3 precursors, VOCs and NOx. • In the summer’s heat and the sun’s strong ultraviolet rays, VOCs and NOx react with atmospheric oxygen (via several steps) to generate O 3.

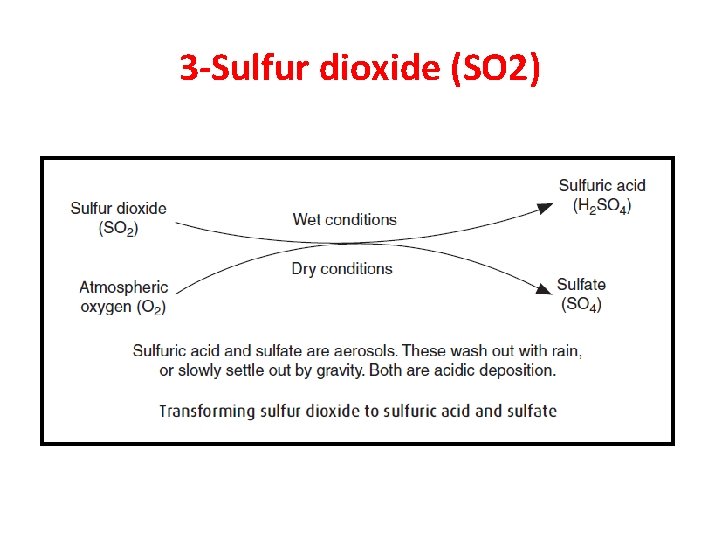
3 -Sulfur dioxide (SO 2) • Sulfur dioxide (SO 2) is a corrosive acid gas, colorless with a sharp irritating odor. • It accounts for ~ 18% of all air pollution, making it second only to CO as the most common urban air pollutant. • It is converted in the atmosphere into substances, which form aerosol. • Sulfuric acid and sulfate are the major aerosols formed.

3 -Sulfur dioxide (SO 2)

Why SO 2 and its acid aerosols are of concern? • Sulfur dioxide reacts with moisture in the eyes, lungs, and other mucous membranes to form strongly irritating acid (about 90% of this conversion occurs in the upper respiratory tract). • Exposure to SO 2 can trigger allergic-type reactions and asthma in sensitive individuals • Sulfur dioxide exposure also aggravates preexisting respiratory or heart disease. • low SO 2 concentrations can damage plants and trees.

Why SO 2 and its acid aerosols are of concern? • Sulfur dioxide’s atmospheric lifetime is only about a day. • However, SO 2 can be converted into tiny particles, largely liquid sulfuric acid and solid sulfate. These tiny particles form aerosols, a gaseous suspension of fine solid or liquid particles, which can remain airborne for many days. The dry particles can slowly settle out; the liquid sulfuric acid can be rained out. • Such tiny aerosols, only 0. 1 to 1 μm in diameter, can be deeply inhaled into the lungs and inflame them.

4 -Nitrogen oxides (NOx) • NOx is the word used for a group of gases, especially nitric oxide (NO) and nitrogen dioxide (NO 2), nitrous oxide (N 2 O). • Nitrogen oxides account for about 6% of US air pollution.

Why NOx gases and their acid aerosols are of concern? • Direct exposure to NOx irritates the lungs, aggravates asthma, and lowers resistance to infection. • Nitrogen dioxide is poisonous to plant life. • As tiny aerosol particles, nitrate and nitric acid, they are deeply inhaled into, and worsen, the lungs. • Nitric acid and nitrate are part of the haze that affects visibility; and contribute to acid deposition. • As aerosols they have a cooling influence on climate. Aerosol particles provide surfaces on which ozonedestroying reactions can occur, contributing to stratospheric-ozone depletion.

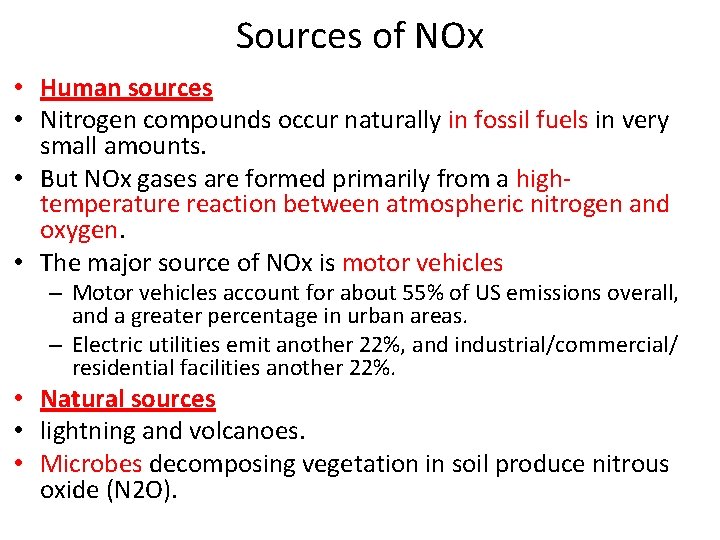
Sources of NOx • Human sources • Nitrogen compounds occur naturally in fossil fuels in very small amounts. • But NOx gases are formed primarily from a hightemperature reaction between atmospheric nitrogen and oxygen. • The major source of NOx is motor vehicles – Motor vehicles account for about 55% of US emissions overall, and a greater percentage in urban areas. – Electric utilities emit another 22%, and industrial/commercial/ residential facilities another 22%. • Natural sources • lightning and volcanoes. • Microbes decomposing vegetation in soil produce nitrous oxide (N 2 O).

References • Hill, M. K. 2010. Understanding environmental pollution. 3 rd Edition. Cambridge University Press. • Radojevic, M. & Bashkin, V. 2007. Practical environmental analysis. 1 st edition. Royal Society of Chemistry. • Raven H. P. &Berg R. L. 2005. Environment. 5 th edition. United States of America: Wiley PLUS.

 Scoreboard computer architecture
Scoreboard computer architecture 11th chemistry thermodynamics lec 13
11th chemistry thermodynamics lec 13 Lec ditto
Lec ditto Scoreboarding computer architecture
Scoreboarding computer architecture Componentes del lec
Componentes del lec 11th chemistry thermodynamics lec 10
11th chemistry thermodynamics lec 10 Biochemistry
Biochemistry August lec 250
August lec 250 Underground pipeline irrigation system
Underground pipeline irrigation system Lec 1
Lec 1 Art 455 lec
Art 455 lec Lec
Lec 132000 lec
132000 lec Xrl in 8051
Xrl in 8051 Tura analítica
Tura analítica Sekisui slec
Sekisui slec 416 lec
416 lec Lec
Lec Brayton cycle
Brayton cycle Lec promotion
Lec promotion Lec anatomia
Lec anatomia Lec hardver
Lec hardver Lec arg
Lec arg Lecsl
Lecsl