First law for a closed system undergoing a

- Slides: 10

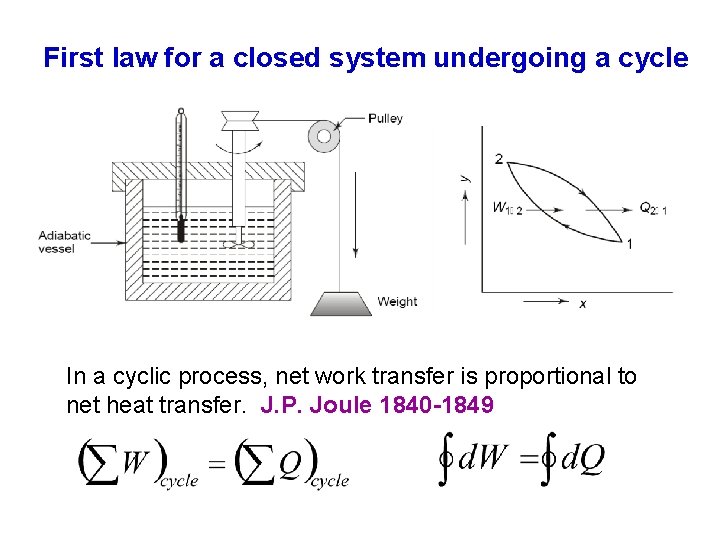
First law for a closed system undergoing a cycle In a cyclic process, net work transfer is proportional to net heat transfer. J. P. Joule 1840 -1849

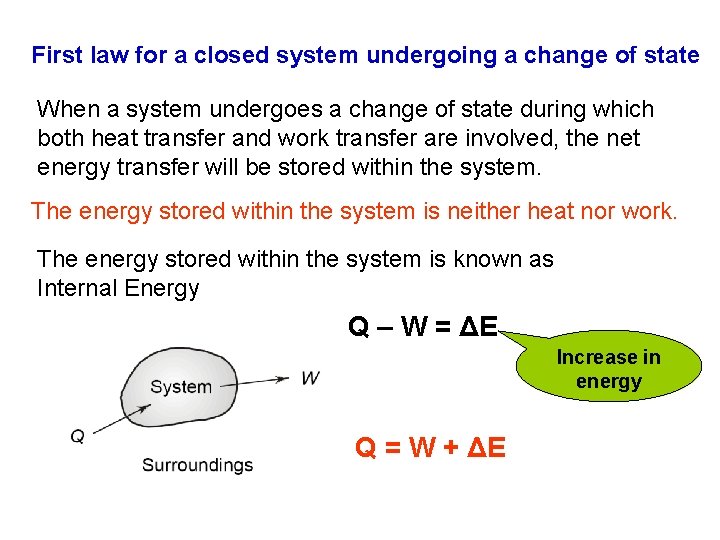
First law for a closed system undergoing a change of state When a system undergoes a change of state during which both heat transfer and work transfer are involved, the net energy transfer will be stored within the system. The energy stored within the system is neither heat nor work. The energy stored within the system is known as Internal Energy Q – W = ΔE Increase in energy Q = W + ΔE

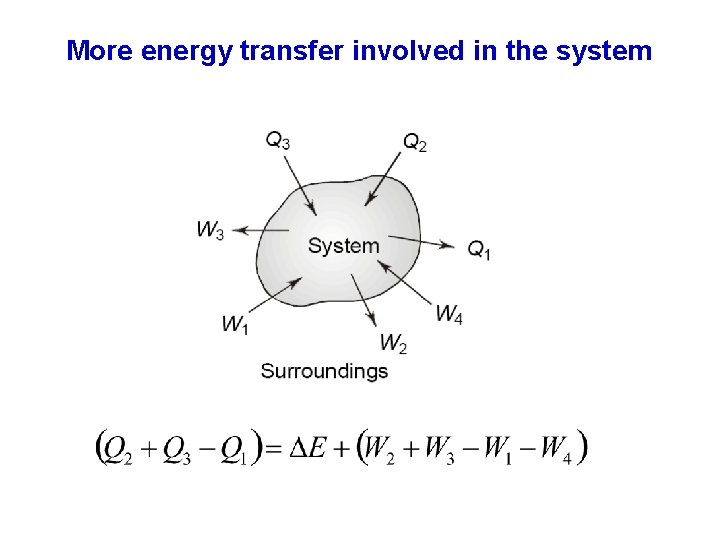
More energy transfer involved in the system

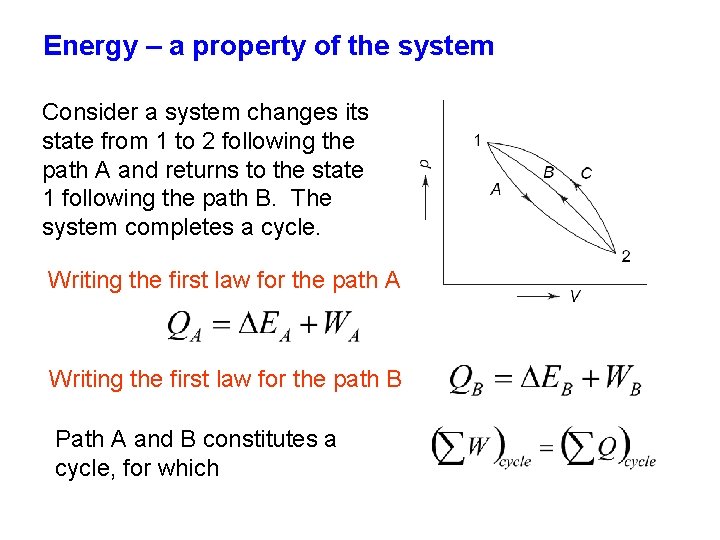
Energy – a property of the system Consider a system changes its state from 1 to 2 following the path A and returns to the state 1 following the path B. The system completes a cycle. Writing the first law for the path A Writing the first law for the path B Path A and B constitutes a cycle, for which

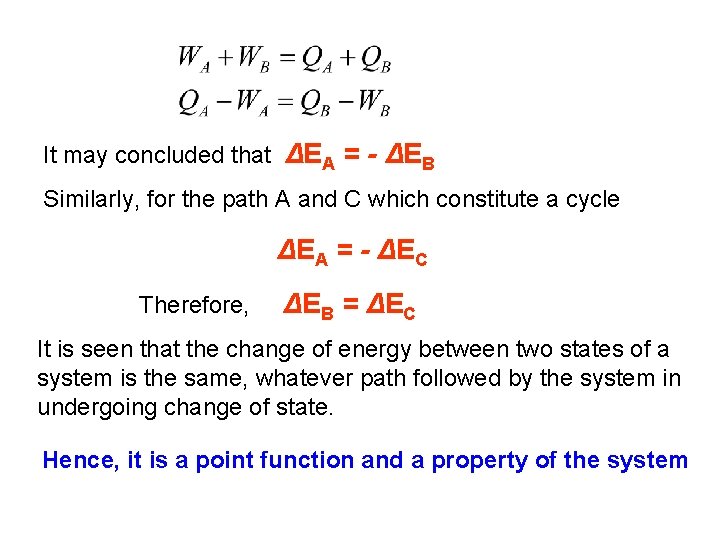
It may concluded that ΔEA = - ΔEB Similarly, for the path A and C which constitute a cycle ΔEA = - ΔEC Therefore, ΔEB = ΔEC It is seen that the change of energy between two states of a system is the same, whatever path followed by the system in undergoing change of state. Hence, it is a point function and a property of the system

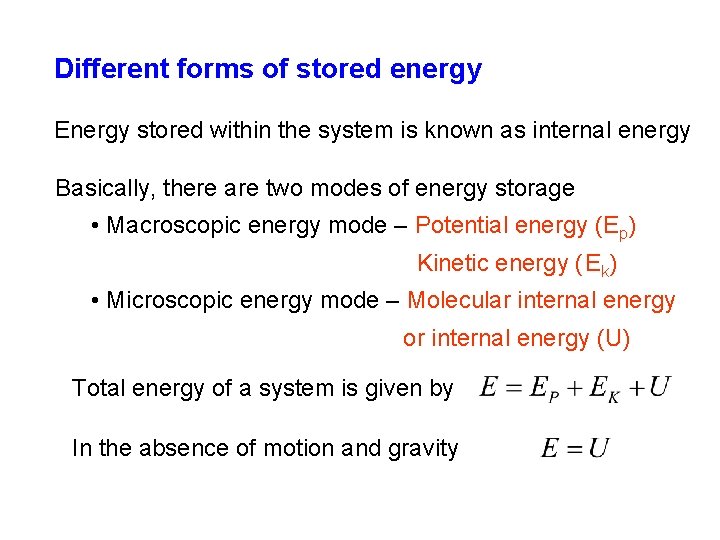
Different forms of stored energy Energy stored within the system is known as internal energy Basically, there are two modes of energy storage • Macroscopic energy mode – Potential energy (Ep) Kinetic energy (Ek) • Microscopic energy mode – Molecular internal energy or internal energy (U) Total energy of a system is given by In the absence of motion and gravity

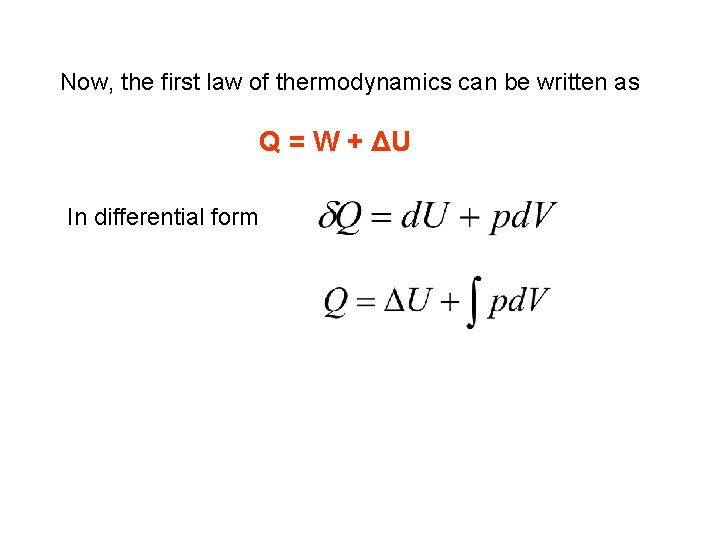
Now, the first law of thermodynamics can be written as Q = W + ΔU In differential form

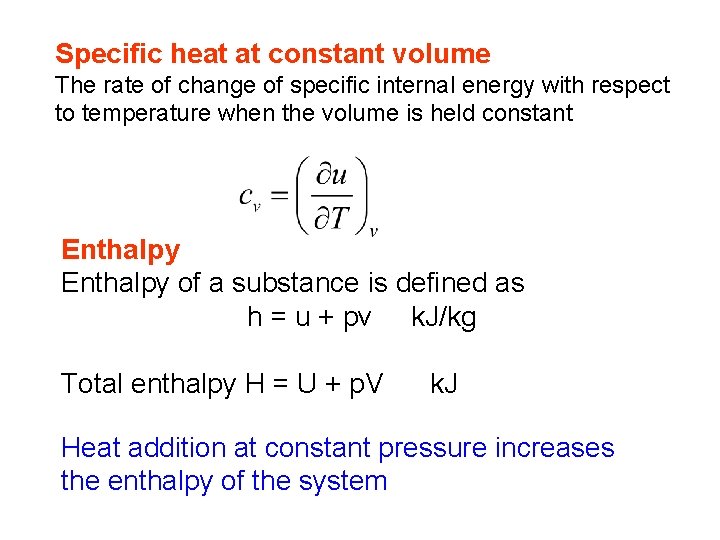
Specific heat at constant volume The rate of change of specific internal energy with respect to temperature when the volume is held constant Enthalpy of a substance is defined as h = u + pv k. J/kg Total enthalpy H = U + p. V k. J Heat addition at constant pressure increases the enthalpy of the system

Specific heat at constant pressure The rate of change of enthalpy with respect to temperature when the pressure is held constant Points to remember Heat addition at constant volume will increase the internal energy Heat addition at constant pressure will increase the enthalpy

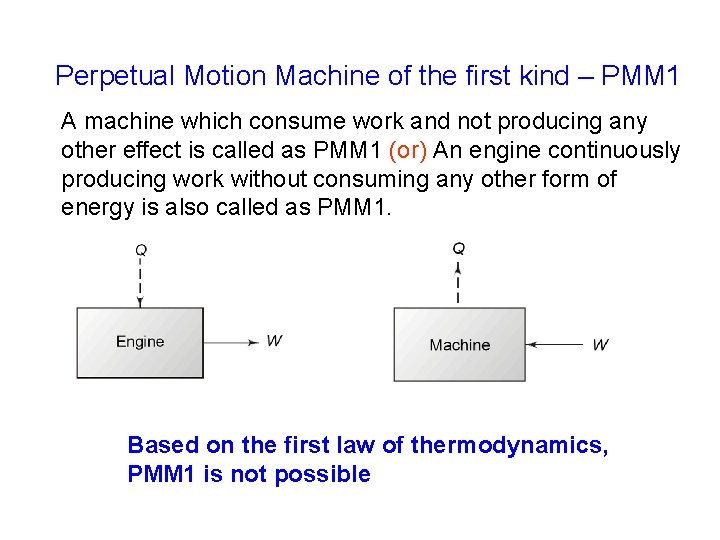
Perpetual Motion Machine of the first kind – PMM 1 A machine which consume work and not producing any other effect is called as PMM 1 (or) An engine continuously producing work without consuming any other form of energy is also called as PMM 1. Based on the first law of thermodynamics, PMM 1 is not possible