First exam Exercises First Exam Exercises 1 Prefixes












- Slides: 39

First exam Exercises

First Exam/ Exercises 1 - Prefixes giga and deci represent, respectively: a) 10 -9 and 10 -1. b) 106 and 10 -3. c) 103 and 10 -3 d) 109 and 10 -1. 2 - If 586 g of bromine occupies 188 m. L, what is the density of bromine in g/m. L? a) 3. 12 g/ml b) 0. 321 g/ml c) 3. 63 g/ml d) 3. 08 g/ml d = m/V m = 586 g, V = 188 m. L d = 586 / 188 = 3. 12 g/ml

First Exam/ Exercises 1 - Prefixes giga and deci represent, respectively: a) 10 -9 and 10 -1. b) 106 and 10 -3. c) 103 and 10 -3 d) 109 and 10 -1. 2 - If 586 g of bromine occupies 188 m. L, what is the density of bromine in g/m. L? a) 3. 12 g/ml b) 0. 321 g/ml c) 3. 63 g/ml d) 3. 08 g/ml 3 - 3. 5 nanometer contains how many micrometers? a) 3. 5 x 10+3 b) 3. 5 x 10 -3 c) 3. 5 x 10+8 d) 3. 5 x 10 -8 First convert from nm to m : 3. 5 X 10 -9 m Then from m to um 3. 5 x 10 -9 x 10+6 = 3. 5 x 10 -3

First Exam/ Exercises 1 - Prefixes giga and deci represent, respectively: a) 10 -9 and 10 -1. b) 106 and 10 -3. c) 103 and 10 -3 d) 109 and 10 -1. 2 - If 586 g of bromine occupies 188 m. L, what is the density of bromine in g/m. L? a) 3. 12 g/ml b) 0. 321 g/ml c) 3. 63 g/ml d) 3. 08 g/ml 3 - 3. 5 nanometer contains how many micrometers? a) 3. 5 x 10+3 b) 3. 5 x 10 -3 c) 3. 5 x 10+8 d) 3. 5 x 10 -8 4 - The element in group 2 A and period 3 is: a) Ga b) Be c) Al d) Mg

First Exam/ Exercises 5 - Predict the formula of a compound formed from Sr and Cl? a) Sr 2 Cl b) Sr 2 Cl 2 c) Sr. Cl 2 d) Sr. Cl Sr+2 Sr. Cl 2 Cl-

First Exam/ Exercises 5 - Predict the formula of a compound formed from Sr and Cl? a) Sr 2 Cl b) Sr 2 Cl 2 c) Sr. Cl 2 d) Sr. Cl 6 - Which compound has the same empirical formula as C 6 H 12 O 6? a) C 12 H 20 O 4 b) C 2 H 8 O 4 c) C 6 H 3 O 6 d) C 12 H 24 O 12 C 6 H 12 O 6 divided by 6 = CH 2 O a- C 12 H 20 O 4 divided by 4 = C 3 H 5 O b- C 2 H 8 O 4 divided by 2 = CH 4 O 2 c- C 6 H 3 O 6 divided by 3 = C 2 HO 2 d- C 12 H 24 O 12 divided by 12 = CH 2 O

First Exam/ Exercises 5 - Predict the formula of a compound formed from Sr and Cl? a) Sr 2 Cl b) Sr 2 Cl 2 c) Sr. Cl 2 d) Sr. Cl 6 - Which compound has the same empirical formula as C 6 H 12 O 6? a) C 12 H 20 O 4 b) C 2 H 8 O 4 c) C 6 H 3 O 6 d) C 12 H 24 O 12 7 - An atom is a) Smallest unit of matter that maintains its chemical identity. b) The smallest unit of a compound. c) Always made of carbon d) Smaller than electron

First Exam/ Exercises 8 -The right answer is: a)Compounds are: N 2, NH 3 , H 2 O and CH 4 b)Elements are: N 2, H 2 O, H 2 and F 2 c) Molecules are: N 2, H 2, F 2 and Cl 2 and Fe 2 SO 4 d) Compounds are: NH 3, O 2, H 2 O and CH 4 9 - Rubidium (Rb) and cesium (Cs) are members of which of the following categories? a) Halogens b) Alkali metals c) Alkaline earth metals d) Noble gases 10 - What is the number of protons, electrons and neutrons in the atom of a) 32 protons, 34 electrons, 16 neutrons c) 16 protons, 18 electrons, 16 neutrons b) 16 protons, 16 electrons, 16 neutrons d) 18 protons, 16 electrons, 32 neutrons

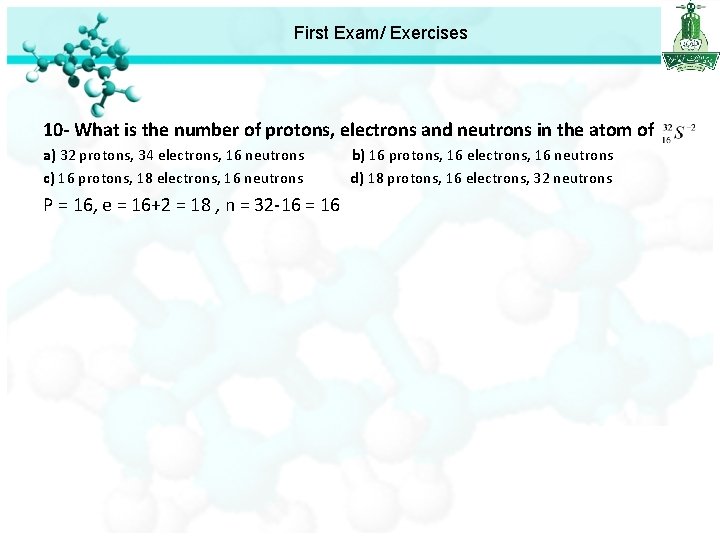
First Exam/ Exercises 10 - What is the number of protons, electrons and neutrons in the atom of a) 32 protons, 34 electrons, 16 neutrons c) 16 protons, 18 electrons, 16 neutrons P = 16, e = 16+2 = 18 , n = 32 -16 = 16 b) 16 protons, 16 electrons, 16 neutrons d) 18 protons, 16 electrons, 32 neutrons

First Exam/ Exercises 8 -The right answer is: a)Compounds are: N 2, NH 3 , H 2 O and CH 4 b)Elements are: N 2, H 2 O, H 2 and F 2 c) Molecules are: N 2, H 2, F 2 and Cl 2 and Fe 2 SO 4 d) Compounds are: NH 3, O 2, H 2 O and CH 4 9 - Rubidium (Rb) and cesium (Cs) are members of which of the following categories? a) Halogens b) Alkali metals c) Alkaline earth metals d) Noble gases 10 - What is the number of protons, electrons and neutrons in the atom of a) 32 protons, 34 electrons, 16 neutrons c) 16 protons, 18 electrons, 16 neutrons b) 16 protons, 16 electrons, 16 neutrons d) 18 protons, 16 electrons, 32 neutrons

First Exam/ Exercises 11 - The correct systematic name for Cr 2 O 3 is a)Chromium (III) oxide. b) Dichromium trioxide. c) Chromium trioxide d) Chromium oxide.

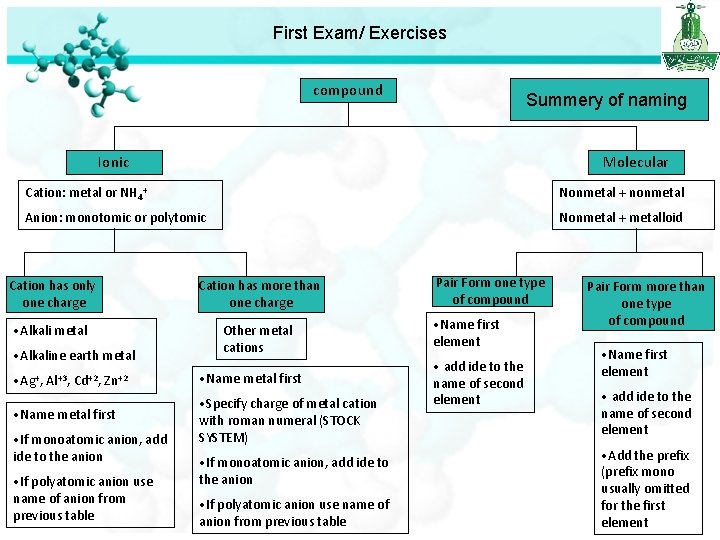
First Exam/ Exercises compound Summery of naming Ionic Molecular Cation: metal or NH 4+ Nonmetal + nonmetal Anion: monotomic or polytomic Nonmetal + metalloid Cation has only one charge Cation has more than one charge • Alkali metal Other metal cations • Alkaline earth metal • Ag+, Al+3, Cd+2, Zn+2 • Name metal first • Specify charge of metal cation with roman numeral (STOCK SYSTEM) • If monoatomic anion, add ide to the anion • If polyatomic anion use name of anion from previous table Pair Form one type of compound • Name first element • add ide to the name of second element Pair Form more than one type of compound • Name first element • add ide to the name of second element • Add the prefix (prefix mono usually omitted for the first element

First Exam/ Exercises 11 - The correct systematic name for Cr 2 O 3 is a)Chromium (III) oxide. b) Dichromium trioxide. c) Chromium trioxide d) Chromium oxide. 12 - The correct formula for calcium sulfate is a) Ca. HSO 4 b) Ca. SO 4 c) Ca. S d) Ca(HSO 4)2 13 - Gallium consists of 60. 108% 69 Ga with a mass of 68. 9256 amu, and 39. 892% 71 Ga with a mass of 70. 9247 amu, the average atomic mass of gallium is: a) 70. 93 amu b) 71. 62 amu c) 68. 93 amu d) 69. 723 amu Average atomic mass = (atomic mass x abundenace)Ga-69+ (atomic mass x abundenace)Ga-71 Average atomic mass = (68. 9256 X (60. 108 /100))Ga-69 + ( 70. 9247 x (39. 892 /100))Ga-71 = 69. 723

First Exam/ Exercises 11 - The correct systematic name for Cr 2 O 3 is a)Chromium (III) oxide. b) Dichromium trioxide. c) Chromium trioxide d) Chromium oxide. 12 - The correct formula for calcium sulfate is a) Ca. HSO 4 b) Ca. SO 4 c) Ca. S d) Ca(HSO 4)2 13 - Gallium consists of 60. 108% 69 Ga with a mass of 68. 9256 amu, and 39. 892% 71 Ga with a mass of 70. 9247 amu, the average atomic mass of gallium is: a) 70. 93 amu b) 71. 62 amu c) 68. 93 amu d) 69. 723 amu 14 - Which pair of Atoms would be most likely to form an ionic compound? a) C and N b) K and Ca c) P and Ar d) Ba and S

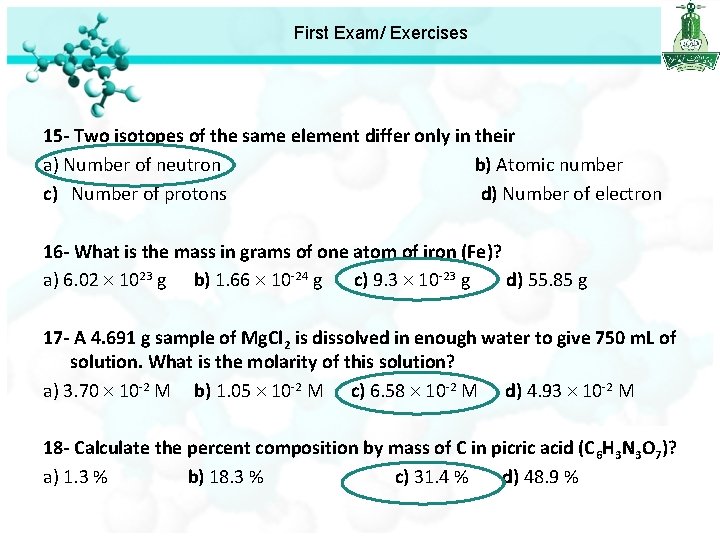
First Exam/ Exercises 15 - Two isotopes of the same element differ only in their a) Number of neutron b) Atomic number c) Number of protons d) Number of electron 16 - What is the mass in grams of one atom of iron (Fe)? a) 6. 02 1023 g b) 1. 66 10 -24 g c) 9. 3 10 -23 g d) 55. 85 g Mass of one atom = atomic mass/ avogadro number = 56 / 6. 022 x 1023 = 9. 3 10 -23 g

First Exam/ Exercises 15 - Two isotopes of the same element differ only in their a) Number of neutron b) Atomic number c) Number of protons d) Number of electron 16 - What is the mass in grams of one atom of iron (Fe)? a) 6. 02 1023 g b) 1. 66 10 -24 g c) 9. 3 10 -23 g d) 55. 85 g 17 - A 4. 691 g sample of Mg. Cl 2 is dissolved in enough water to give 750 m. L of solution. What is the molarity of this solution? a) 3. 70 10 -2 M b) 1. 05 10 -2 M c) 6. 58 10 -2 M d) 4. 93 10 -2 M M = n/V n = mass /molar mass = 4. 691 / 95= 0. 0493 mole M = 0. 0493 / (750 / 1000)= 0. 0658 M = 6. 58 X 10 -2 M

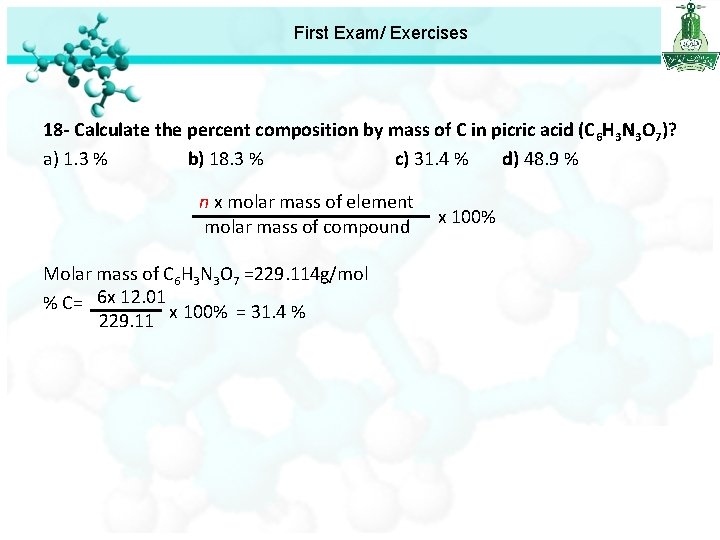
First Exam/ Exercises 15 - Two isotopes of the same element differ only in their a) Number of neutron b) Atomic number c) Number of protons d) Number of electron 16 - What is the mass in grams of one atom of iron (Fe)? a) 6. 02 1023 g b) 1. 66 10 -24 g c) 9. 3 10 -23 g d) 55. 85 g 17 - A 4. 691 g sample of Mg. Cl 2 is dissolved in enough water to give 750 m. L of solution. What is the molarity of this solution? a) 3. 70 10 -2 M b) 1. 05 10 -2 M c) 6. 58 10 -2 M d) 4. 93 10 -2 M 18 - Calculate the percent composition by mass of C in picric acid (C 6 H 3 N 3 O 7)? a) 1. 3 % b) 18. 3 % c) 31. 4 % d) 48. 9 %

First Exam/ Exercises 18 - Calculate the percent composition by mass of C in picric acid (C 6 H 3 N 3 O 7)? a) 1. 3 % b) 18. 3 % c) 31. 4 % d) 48. 9 % n x molar mass of element molar mass of compound Molar mass of C 6 H 3 N 3 O 7 =229. 114 g/mol % C= 6 x 12. 01 x 100% = 31. 4 % 229. 11 x 100%

First Exam/ Exercises 15 - Two isotopes of the same element differ only in their a) Number of neutron b) Atomic number c) Number of protons d) Number of electron 16 - What is the mass in grams of one atom of iron (Fe)? a) 6. 02 1023 g b) 1. 66 10 -24 g c) 9. 3 10 -23 g d) 55. 85 g 17 - A 4. 691 g sample of Mg. Cl 2 is dissolved in enough water to give 750 m. L of solution. What is the molarity of this solution? a) 3. 70 10 -2 M b) 1. 05 10 -2 M c) 6. 58 10 -2 M d) 4. 93 10 -2 M 18 - Calculate the percent composition by mass of C in picric acid (C 6 H 3 N 3 O 7)? a) 1. 3 % b) 18. 3 % c) 31. 4 % d) 48. 9 %

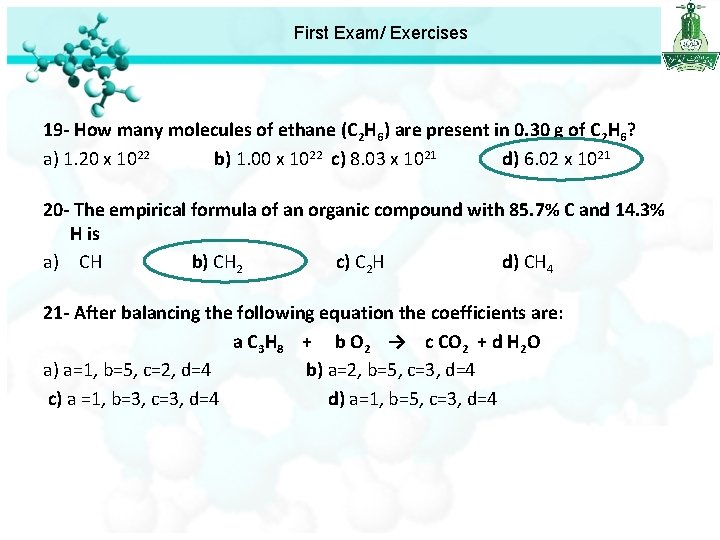
First Exam/ Exercises 19 - How many molecules of ethane (C 2 H 6) are present in 0. 30 g of C 2 H 6? a) 1. 20 x 1022 b) 1. 00 x 1022 c) 8. 03 x 1021 d) 6. 02 x 1021 Molar mass of ethane = 2 x 12 + 6 x 1 = 30 g/mole First we should calculate the number of mole Number of mole = mass / molar mass Number of mole = 0. 30 / 30 = 0. 01 mole We know that Number of molecules = avogadro’s number x number of mole = 6. 022 x 1023 x 0. 01 = 6. 02 x 1021 molecules.

First Exam/ Exercises 19 - How many molecules of ethane (C 2 H 6) are present in 0. 30 g of C 2 H 6? a) 1. 20 x 1022 b) 1. 00 x 1022 c) 8. 03 x 1021 d) 6. 02 x 1021 20 - The empirical formula of an organic compound with 85. 7% C and 14. 3% H is a) CH b) CH 2 c) C 2 H d) CH 4 Divided by the smallest number 1 - we change from % to g of mole which is 7. 14 85. 7 g of C, 14. 3 g of H 7. 14 14. 3 H: =1 =2 2 - change from g to mole using C: 7. 14 85. 7 Thus the empirical formula is CH 2 = 7. 14 mol of C nc = 12 14. 3 = 14. 3 mol of H n. H = 1

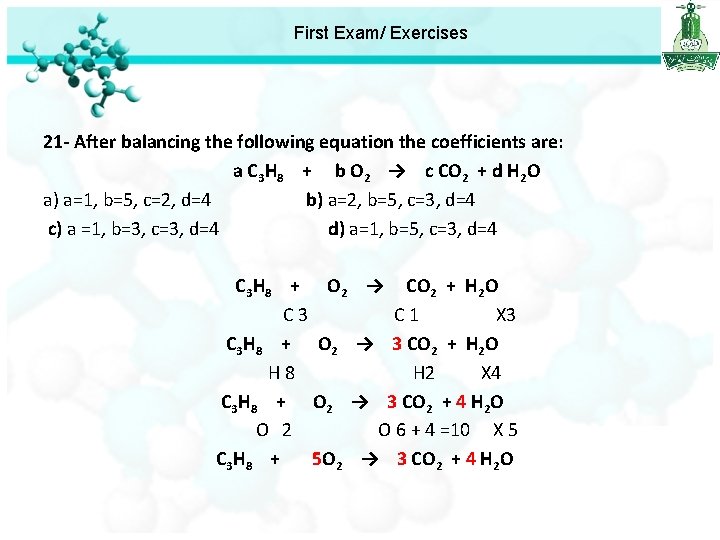
First Exam/ Exercises 19 - How many molecules of ethane (C 2 H 6) are present in 0. 30 g of C 2 H 6? a) 1. 20 x 1022 b) 1. 00 x 1022 c) 8. 03 x 1021 d) 6. 02 x 1021 20 - The empirical formula of an organic compound with 85. 7% C and 14. 3% H is a) CH b) CH 2 c) C 2 H d) CH 4 21 - After balancing the following equation the coefficients are: a C 3 H 8 + b O 2 → c CO 2 + d H 2 O a) a=1, b=5, c=2, d=4 b) a=2, b=5, c=3, d=4 c) a =1, b=3, c=3, d=4 d) a=1, b=5, c=3, d=4

First Exam/ Exercises 21 - After balancing the following equation the coefficients are: a C 3 H 8 + b O 2 → c CO 2 + d H 2 O a) a=1, b=5, c=2, d=4 b) a=2, b=5, c=3, d=4 c) a =1, b=3, c=3, d=4 d) a=1, b=5, c=3, d=4 C 3 H 8 + O 2 → CO 2 + H 2 O C 3 C 1 X 3 C 3 H 8 + O 2 → 3 CO 2 + H 2 O H 8 H 2 X 4 C 3 H 8 + O 2 → 3 CO 2 + 4 H 2 O O 2 O 6 + 4 =10 X 5 C 3 H 8 + 5 O 2 → 3 CO 2 + 4 H 2 O

First Exam/ Exercises 19 - How many molecules of ethane (C 2 H 6) are present in 0. 30 g of C 2 H 6? a) 1. 20 x 1022 b) 1. 00 x 1022 c) 8. 03 x 1021 d) 6. 02 x 1021 20 - The empirical formula of an organic compound with 85. 7% C and 14. 3% H is a) CH b) CH 2 c) C 2 H d) CH 4 21 - After balancing the following equation the coefficients are: a C 3 H 8 + b O 2 → c CO 2 + d H 2 O a) a=1, b=5, c=2, d=4 b) a=2, b=5, c=3, d=4 c) a =1, b=3, c=3, d=4 d) a=1, b=5, c=3, d=4 22 - Calculate the number of O atoms in 2. 50 g of sucrose (C 12 H 22 O 11) a) 3. 87 x 1022 b) 4. 84 x 1022 c) 5. 81 x 1022 d) 6. 78 x 1022

First Exam/ Exercises 22 - Calculate the number of O atoms in 2. 50 g of sucrose (C 12 H 22 O 11) a) 3. 87 x 1022 b) 4. 84 x 1022 c) 5. 81 x 1022 d) 6. 78 x 1022 First we calculate the number of mole n = 2. 5/ 342 = 0. 007 mole Number of molecules = Avogadro's number x number of mole = 6. 022 x 1023 x 0. 007 = 4. 4 x 1022 molecules From the chemical formula of sucrose C 12 H 22 O 11 1 molecules of sucrose = 11 atom of O 4. 4 x 1021 molecules = ? Atom of O 11 x 4. 4 x 1021 = 4. 84 x 1021 atoms

First Exam/ Exercises 19 - How many molecules of ethane (C 2 H 6) are present in 0. 30 g of C 2 H 6? a) 1. 20 x 1022 b) 1. 00 x 1022 c) 8. 03 x 1021 d) 6. 02 x 1021 20 - The empirical formula of an organic compound with 85. 7% C and 14. 3% H is a) CH b) CH 2 c) C 2 H d) CH 4 21 - After balancing the following equation the coefficients are: a C 3 H 8 + b O 2 → c CO 2 + d H 2 O a) a=1, b=5, c=2, d=4 b) a=2, b=5, c=3, d=4 c) a =1, b=3, c=3, d=4 d) a=1, b=5, c=3, d=4 22 - Calculate the number of O atoms in 2. 50 g of sucrose (C 12 H 22 O 11) a) 3. 87 x 1022 b) 4. 84 x 1022 c) 5. 81 x 1022 d) 6. 78 x 1022

First Exam/ Exercises 23 - An empirical formula of an organic compound is C 3 H 4 O 2 , if the molecular weight of the compound is (360 g/mol), the molecular formula of the compound will be: a) C 6 H 8 O 4 b) C 12 H 16 O 8 c) C 9 H 12 O 6 d) C 15 H 20 O 10 FIRST we calculate the molar mass of emperical formula C 3 H 4 O 2 = 72 g/mol Ratio = 360 / 72 = 5 molecular formula = ratio x empirical formula = 5 x C 3 H 4 O 2 = C 15 H 20 O 10

First Exam/ Exercises 23 - An empirical formula of an organic compound is C 3 H 4 O 2 , if the molecular weight of the compound is (360 g/mol), the molecular formula of the compound will be: a) C 6 H 8 O 4 b) C 12 H 16 O 8 c) C 9 H 12 O 6 d) C 15 H 20 O 10 24 - How many moles of Fe. SO 4 are present in a 500 mg of this salt? a) 1. 64 x 10 -3 b) 2. 30 x 10 -3 c) 3. 29 x 10 -3 d) 4. 93 x 10 -3 Mass = 500 mg = 0. 5 g n= mass / molar mass = 0. 5 / 152 = 0. 00329 = 3. 29 x 10 -3 g

First Exam/ Exercises 23 - An empirical formula of an organic compound is C 3 H 4 O 2 , if the molecular weight of the compound is (360 g/mol), the molecular formula of the compound will be: a) C 6 H 8 O 4 b) C 12 H 16 O 8 c) C 9 H 12 O 6 d) C 15 H 20 O 10 24 - How many moles of Fe. SO 4 are present in a 500 mg of this salt? a) 1. 64 x 10 -3 b) 2. 30 x 10 -3 c) 3. 29 x 10 -3 d) 4. 93 x 10 -3 25 - How many milliliter of water must be added to 200 m. L of 0. 15 M Na 2 CO 3 to prepare 0. 05 M Na 2 CO 3? a) 200 m. L b) 300 m. L c) 400 m. L d) 450 m. L M 1 V 1 = M 2 V 2 0. 15 X 200 = 0. 05 X V 2 = 0. 15 X 200 / 0. 05 = 600 ml Add water = 600 -200 = 400 ml

First Exam/ Exercises 23 - An empirical formula of an organic compound is C 3 H 4 O 2 , if the molecular weight of the compound is (360 g/mol), the molecular formula of the compound will be: a) C 6 H 8 O 4 b) C 12 H 16 O 8 c) C 9 H 12 O 6 d) C 15 H 20 O 10 24 - How many moles of Fe. SO 4 are present in a 500 mg of this salt? a) 1. 64 x 10 -3 b) 2. 30 x 10 -3 c) 3. 29 x 10 -3 d) 4. 93 x 10 -3 25 - How many milliliter of water must be added to 200 m. L of 0. 15 M Na 2 CO 3 to prepare 0. 05 M Na 2 CO 3? a) 200 m. L b) 300 m. L c) 400 m. L d) 450 m. L

First Exam/ Exercises 26 - What is theoretical yield of chromium (Cr) that can be produced by the reaction of 40. 0 g of Cr 2 O 3 with 8. 00 g of aluminum (Al) according to the chemical reaction? 2 Al + Cr 2 O 3 Al 2 O 3 + 2 Cr a) 15. 4 g b) 17. 33 g c) 19. 4 g d) 13. 48 g First we have to determine the limiting reagent: First start with Al 1 -Convert to mole : n = 8 / 27 = 0. 296 mol 2 - from equation 2 mole Al ===== 2 mole Cr 0. 296 mole Al =====? Mole Cr 2 x 0. 296 = 2 x ? Mole of Cr = 0. 296 mol Mass = n x molar mass = 0. 296 x 52 =15. 4 g second start with Cr 2 O 3 1 -Convert to mole : n = 40 / 152 = 0. 263 mol 2 - from equation 1 mole Cr 2 O 3 ===== 2 mole Cr 0. 263 mole Cr 2 O 3 =====? Mole Cr 2 x 0. 263 = 1 x ? Mole of Cr = 0. 526 mol

First Exam/ Exercises 26 - What is theoretical yield of chromium (Cr) that can be produced by the reaction of 40. 0 g of Cr 2 O 3 with 8. 00 g of aluminum (Al) according to the chemical reaction? 2 Al + Cr 2 O 3 Al 2 O 3 + 2 Cr a) 15. 4 g b) 17. 33 g c) 19. 4 g d) 13. 48 g 27 - If the actual yield for the experiment in question (26) produced 13. 0 g, what is the percentage yield? a) 84. 4% b) 75. 0% c) 67% d) 96. 4%

First Exam/ Exercises 26 - What is theoretical yield of chromium (Cr) that can be produced by the reaction of 40. 0 g of Cr 2 O 3 with 8. 00 g of aluminum (Al) according to the chemical reaction? 2 Al + Cr 2 O 3 Al 2 O 3 + 2 Cr a) 15. 4 g b) 17. 33 g c) 19. 4 g d) 13. 48 g 27 - If the actual yield for the experiment in question (26) produced 13. 0 g, what is the percentage yield? a) 84. 4% b) 75. 0% c) 67% d) 96. 4% 28 - The mass of Na that will react with excess of water to produce 16. 0 g Na. OH is 2 Na(s) + 2 H 2 O(l) → H 2(g) + 2 Na. OH(aq) a) 5. 75 g b) 6. 90 g c) 8. 05 g d) 9. 20 g

First Exam/ Exercises 28 - The mass of Na that will react with excess of water to produce 16. 0 g Na. OH is 2 Na(s) + 2 H 2 O(l) → H 2(g) + 2 Na. OH(aq) a) 5. 75 g b) 6. 90 g c) 8. 05 g d) 9. 20 g 1 -First make sure the equation is balanced 2 - g to mole n = mass / molar mass = 16 / 40 = 0. 4 mol From equation 2 mole Na =======2 mole Na. OH ? mole Na ==== 0. 4 Mole Na. OH 2 X 0. 4 = 2 X ? Mole of Na = 0. 4 mole Mass = n x molar mass = 0. 4 x 23 = 9. 2 g

First Exam/ Exercises 26 - What is theoretical yield of chromium (Cr) that can be produced by the reaction of 40. 0 g of Cr 2 O 3 with 8. 00 g of aluminum (Al) according to the chemical reaction? 2 Al + Cr 2 O 3 Al 2 O 3 + 2 Cr a) 15. 4 g b) 17. 33 g c) 19. 4 g d) 13. 48 g 27 - If the actual yield for the experiment in question (26) produced 13. 0 g, what is the percentage yield? a) 84. 4% b) 75. 0% c) 67% d) 96. 4% 28 - The mass of Na that will react with excess of water to produce 16. 0 g Na. OH is 2 Na(s) + 2 H 2 O(l) → H 2(g) + 2 Na. OH(aq) a) 5. 75 g b) 6. 90 g c) 8. 05 g d) 9. 20 g

First Exam/ Exercises 29 - A 100. 0 m. L of 0. 25 M HCl is diluted with water to a total volume of 200. 0 m. L. What is the final concentration in the resulting solution? a) 0. 125 M b) 0. 083 M c) 0. 05 M d) 0. 0625 M M 1 V 1 = M 2 V 2 0. 25 X 100 = M 2 X 200 M 2= 0. 25 X 100 / 200 = 0. 125 M

First Exam/ Exercises 29 - A 100. 0 m. L of 0. 25 M HCl is diluted with water to a total volume of 200. 0 m. L. What is the final concentration in the resulting solution? a) 0. 125 M b) 0. 083 M c) 0. 05 M d) 0. 0625 M 30 - What is the mass of carbon in 10 g carbon dioxide (CO 2)? a) 4. 1 g b) 2. 73 g c) 6. 82 g d) 5. 45 g Mole of CO 2 = mass / molar mass = 10 / 44 = 0. 227 mol From the formula 1 mole C ====== 1 mol CO 2 ? Mole C ====== 0. 227 mole CO 2 Mole of C = 0. 227 mol Mass of C = n x molar mass = 0. 227 x 12 = 2. 73 g.

First Exam/ Exercises 29 - A 100. 0 m. L of 0. 25 M HCl is diluted with water to a total volume of 200. 0 m. L. What is the final concentration in the resulting solution? a) 0. 125 M b) 0. 083 M c) 0. 05 M d) 0. 0625 M 30 - What is the mass of carbon in 10 g carbon dioxide (CO 2)? a) 4. 1 g b) 2. 73 g c) 6. 82 g d) 5. 45 g
