Fires and Explosions Fires and Explosions t Definitions


























- Slides: 40

Fires and Explosions

Fires and Explosions t Definitions t Flammability t Flash Points t Flammability limits t Mixtures t Temperature Dependence t Pressure Dependence t Minimum Oxygen Concentration t Minimum Ignition Energy t Adiabatic Compression t Ignition Sources

Introduction We have been talking about source models for the release of materials and about dispersion models if the material is a toxicant. t. Another concern is a release of flammable materials where we need to worry about fires and explosions. t

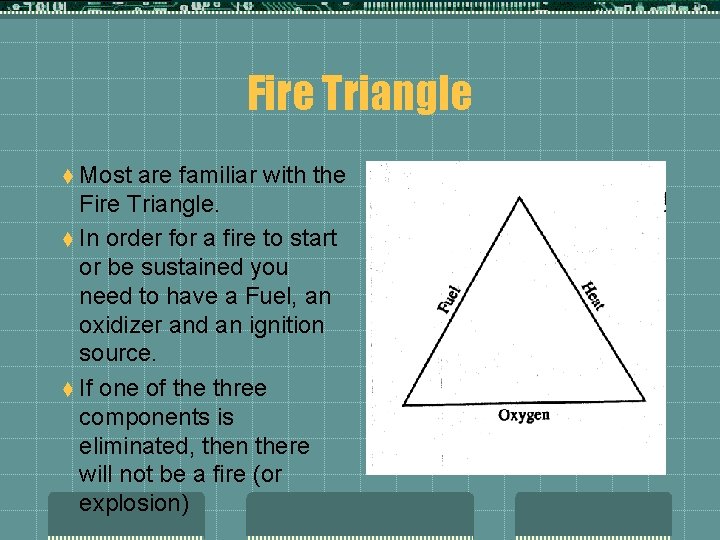
Fire Triangle t Most are familiar with the Fire Triangle. t In order for a fire to start or be sustained you need to have a Fuel, an oxidizer and an ignition source. t If one of the three components is eliminated, then there will not be a fire (or explosion)

Fuel t Fuel must be present in certain concentrations. t Typical cases where fuel occur are if there is a leak, during filling operations, transfer operations, or excessive dusts. t Although we often cannot always eliminate these sources we can help by having good ventilation to keep vapors from building up. t Often we locate things out-doors, use grating on floors so vapors don’t build up.

Oxidizers t. Oxygen is the most common oxidizer, especially that found in ambient air. t. For oxygen, we often use “inerting” with nitrogen, helium blankets over flammable materials to reduce O 2 content below that where you can have combustion.

Ignition Sources t. Heat is a common ignition source. t“Ignition sources are free!!!” t. Although we can eliminate ignition sources, it is almost inevitable that an ignition source will be available if there is a large release of flammable material that cannot be diluted quickly.

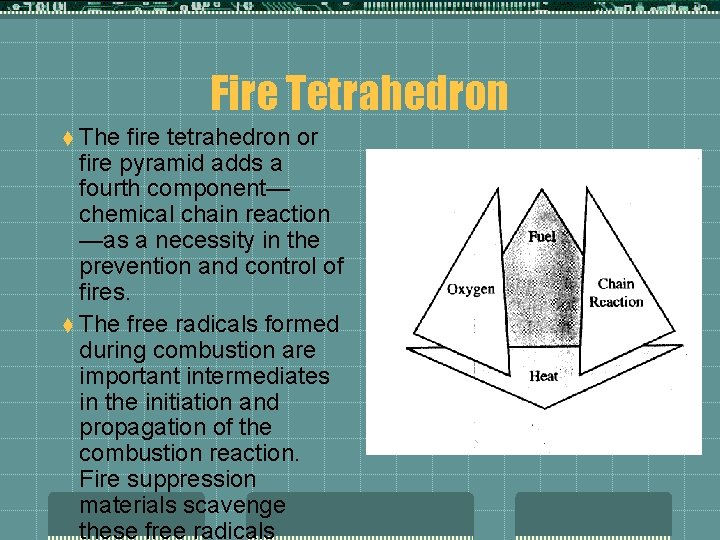
Fire Tetrahedron t The fire tetrahedron or fire pyramid adds a fourth component— chemical chain reaction —as a necessity in the prevention and control of fires. t The free radicals formed during combustion are important intermediates in the initiation and propagation of the combustion reaction. Fire suppression materials scavenge these free radicals

Definitions t. Combustion – a chemical reaction in which a substance combines with an oxidizer and releases energy. t. Explosion – rapid expansion of gases resulting in a rapid moving pressure or shock wave. t. Mechanical Explosion – due to failure of vessel with high pressure non reactive gas.

Explosions t Detonation – explosion (chemical reaction) with shock wave greater than speed of sound t Deflagration – explosion (chemical reaction) with shock wave less than speed of sound t BLEVE – Boiling Liquid Expanding Vapor Explosion – when liquid is at a temperature above its atmospheric boiling point. Vessel ruptures – flammable liquid flashes and results in a fire/explosion

Explosions t. Confined explosion – an explosion occurring within a vessel or a building. Usually results in injury to the building inhabitants and extensive damage. t. Unconfined explosion – an explosion occurring in the open. Usually results from spill of a flammable gas spill. These explosions are rarer than confined since dilution occurs.

Explosions Dust Explosions - This explosion results from the rapid combustion of fine solid particles. Many solid materials become very flammable when reduced to a fine powder. t

Fires and Explosions t Definitions t Flammability t Flash Point t Flammability limits t Mixtures t Temperature Dependence t Pressure Dependence t Minimum Oxygen Concentration t Minimum Ignition Energy t Adiabatic Compression t Ignition Sources

Flammability t. Flash Point (FP) – a property of material used to determine the fire and explosive hazard. The lowest temperature of a liquid at which it gives off enough vapor to form an ignitable mixture with air. t. Needs to be determined experimentally. t. Different methods to determine, open cup and closed cup. Open cup is usually a few degrees higher.

National Fire Protection Association Flammability classification t. Flammable IA – Flash point < 73°F, boiling point < 100 °F t. Flammable IB – Flash point < 73°F, boiling point > 100 °F t. Flammable IC – 73°F < Flash point < 100 °F t. Combustible II – 100 °F < Flash point < 140 °F t. Combustible IIIA – 140 °F < Flash point < 200 °F t. Combustible IIIB – Flash point > 200 °F

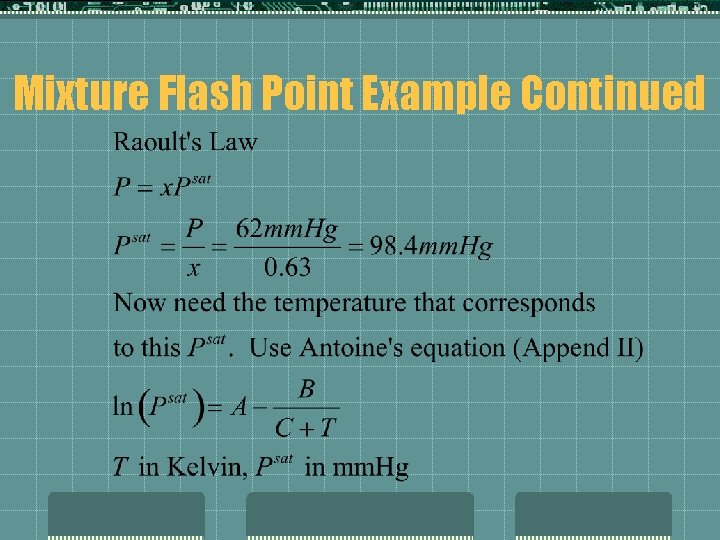
Mixture Flash Points t. Flash Points of mixtures can be estimated only IF one of the components is flammable. If more than one is flammable then need to determine experimentally. t. For mixtures: t. Determine the temperature at which the vapor pressure of the flammable in the liquid is equal to the pure component vapor pressure at its flash point.

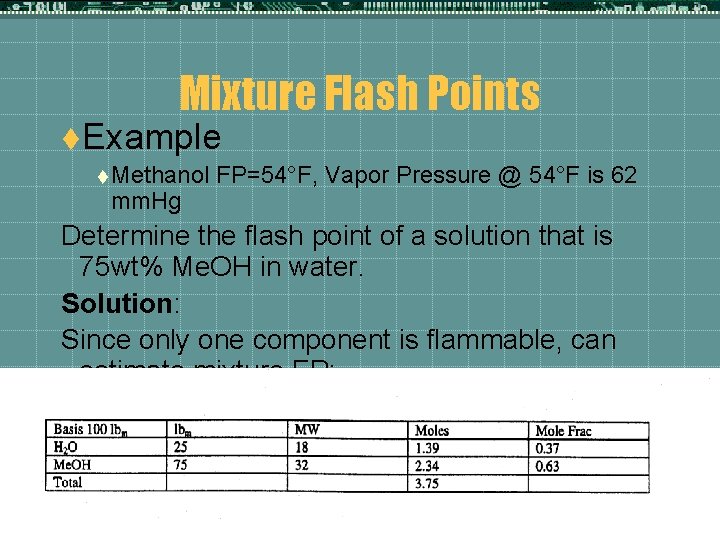
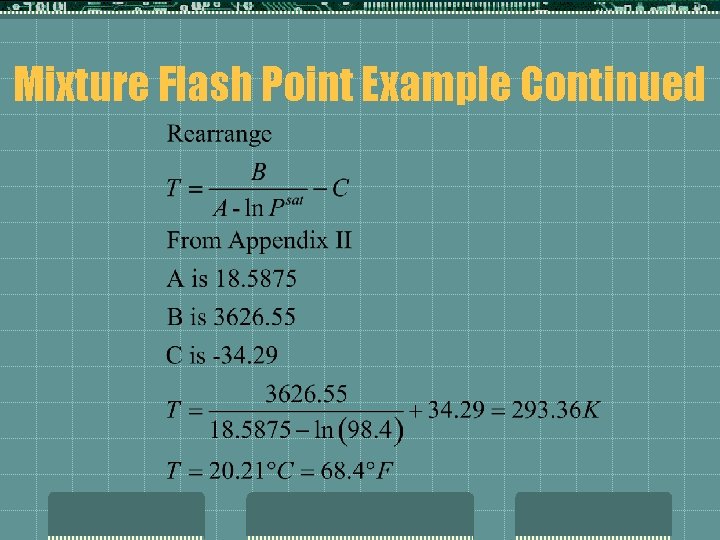
Mixture Flash Points t. Example t Methanol mm. Hg FP=54°F, Vapor Pressure @ 54°F is 62 Determine the flash point of a solution that is 75 wt% Me. OH in water. Solution: Since only one component is flammable, can estimate mixture FP:

Mixture Flash Point Example Continued

Mixture Flash Point Example Continued

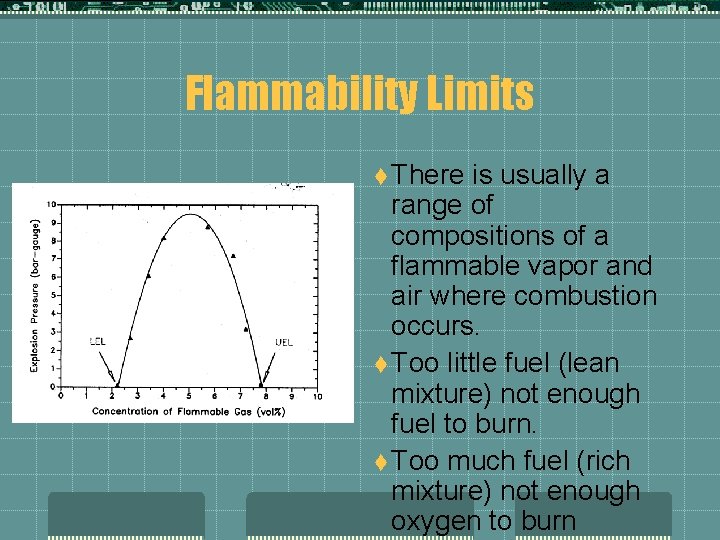
Flammability Limits t There is usually a range of compositions of a flammable vapor and air where combustion occurs. t Too little fuel (lean mixture) not enough fuel to burn. t Too much fuel (rich mixture) not enough oxygen to burn

Flammability Limits t. Table 6 -1 gives upper flammability limits and lower flammability limits for several common substances. t. Experimentally determined. t. LFL can be estimated from Flash Point: .

Mixture Flammability Limits t. If you have a mixture of flammable components you can calculate Lower Flammability Limit of the mixture LFLmix using Le Chatelier’s relationship:

Mixture Flammability Limits t. You can also calculate an Upper Flammability Limit of the mixture UFLmix using Le Chatelier’s relationship:

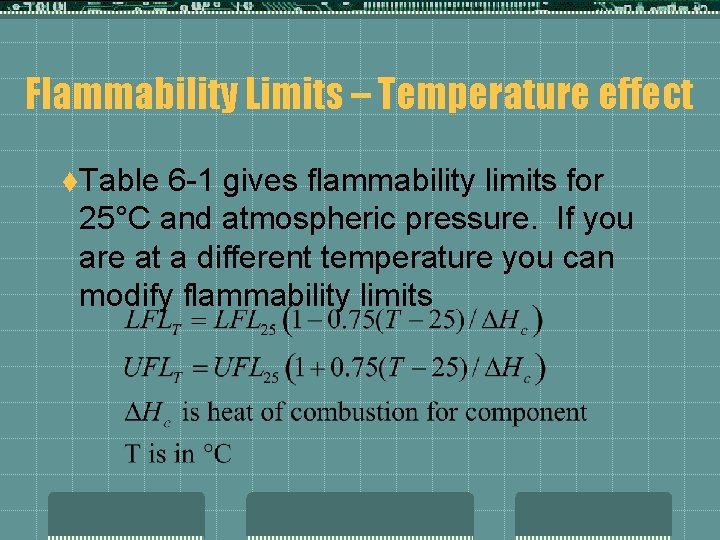
Flammability Limits – Temperature effect t. Table 6 -1 gives flammability limits for 25°C and atmospheric pressure. If you are at a different temperature you can modify flammability limits

Flammability Limits – Pressure effects t t LFL is not affected by pressure UFL does depend on the pressure t Procedure t Correct for Temperature t Correct for Pressure t Calculate for mixture

Fires and Explosions t Definitions t Flammability t Flash Points t Flammability limits t Mixtures t Temperature Dependence t Pressure Dependence t Minimum Oxygen Concentration t Minimum Ignition Energy t Adiabatic Compression t Ignition Sources

Minimum Oxygen Concentration (MOC) LFL is based on “air” but actually it is O 2 that is important. Often in industry they “inert” to dilute the O 2 concentration. t Below the MOC the reaction cannot generate enough energy to heat the entire mixture to the extent required for self propagation. t

MOC

Fires and Explosions t Definitions t Flammability t Flash Points t Flammability limits t Mixtures t Temperature Dependence t Pressure Dependence t Minimum Oxygen Concentration t Minimum Ignition Energy t Adiabatic Compression t Ignition Sources

Minimum Ignition Energy (MIE) t. Minimum energy input needed to initiate combustion t. Most hydrocarbons have low MIE~0. 25 m. J t. Whereas the “spark” from walking across the room is 22 m. J (almost 100 X too much) t. Again, we always assume that an ignition source will exist t. Table 6 -2 gives MIEs for some substances

Fires and Explosions t Definitions t Flammability t Flash Points t Flammability limits t Mixtures t Temperature Dependence t Pressure Dependence t Minimum Oxygen Concentration t Minimum Ignition Energy t Adiabatic Compression t Ignition Sources

Adiabatic Compression When gases are compressed they heat up and can ignite (this is how a diesel engine works, also the cause of “knocking” in gasoline engines) t. The adiabatic temperature rise is: t

Fires and Explosions t Definitions t Flammability t Flash Points t Flammability limits t Mixtures t Temperature Dependence t Pressure Dependence t Minimum Oxygen Concentration t Minimum Ignition Energy t Adiabatic Compression t Ignition Sources

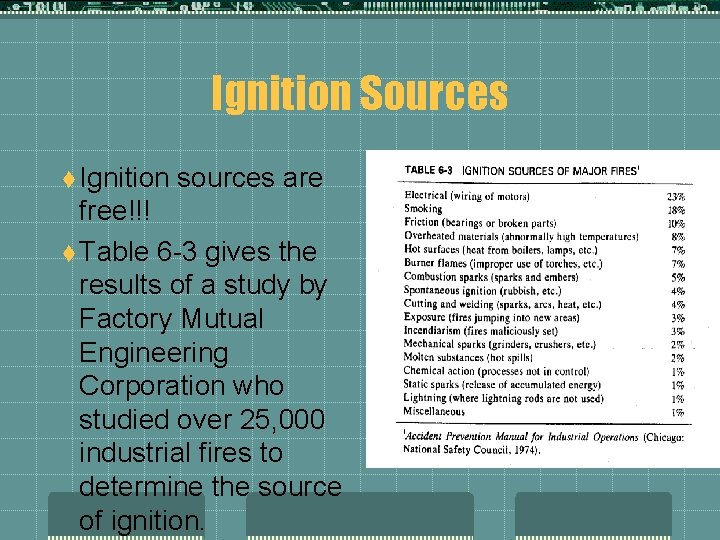
Ignition Sources t Ignition sources are free!!! t Table 6 -3 gives the results of a study by Factory Mutual Engineering Corporation who studied over 25, 000 industrial fires to determine the source of ignition.

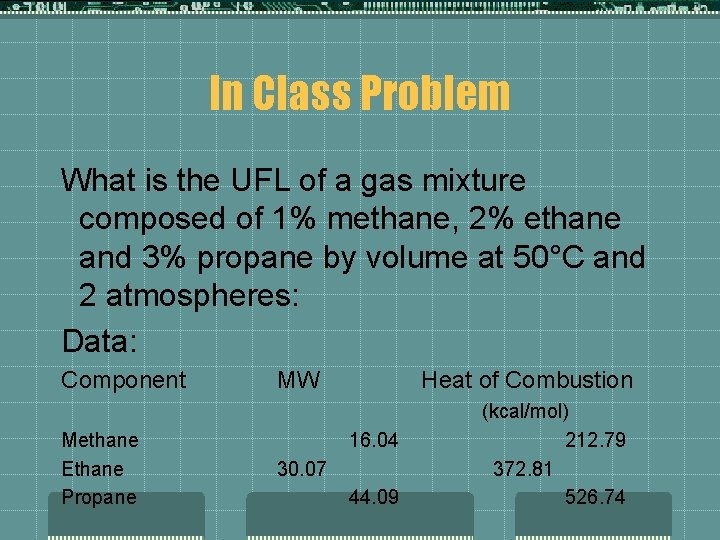
In Class Problem What is the UFL of a gas mixture composed of 1% methane, 2% ethane and 3% propane by volume at 50°C and 2 atmospheres: Data: Component Methane Ethane Propane MW Heat of Combustion 16. 04 30. 07 44. 09 (kcal/mol) 212. 79 372. 81 526. 74

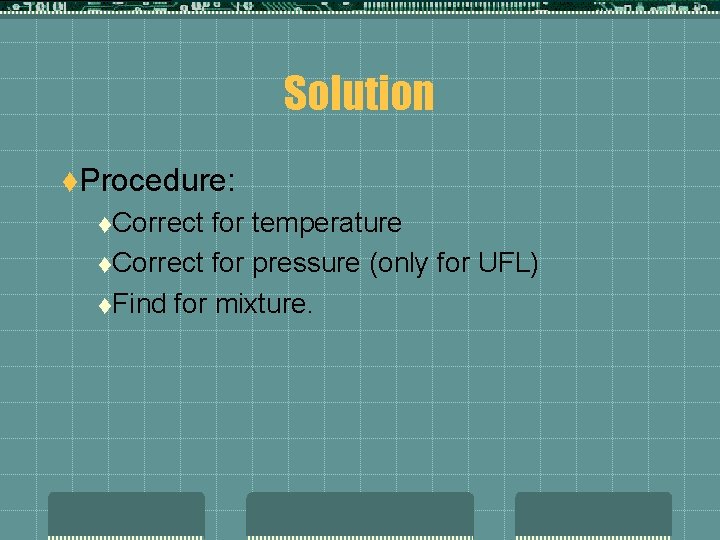
Solution t. Procedure: t. Correct for temperature t. Correct for pressure (only for UFL) t. Find for mixture.

Solution Correction for Temperature : UFL from Table 6 -1 t

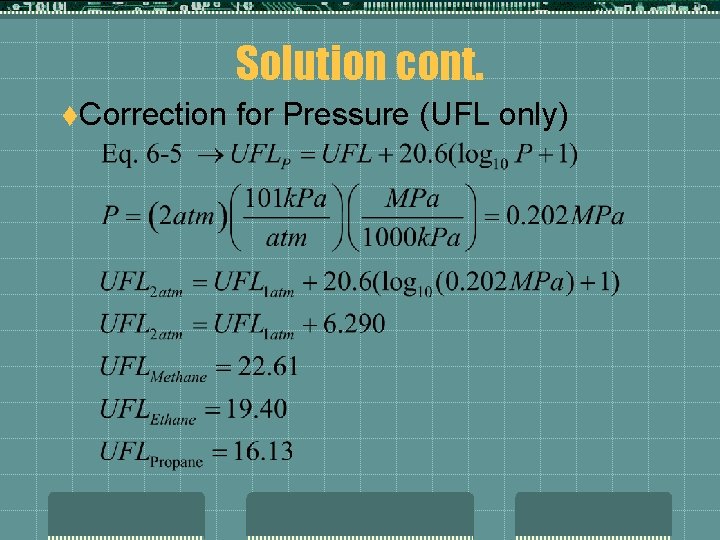
Solution cont. t. Correction for Pressure (UFL only)

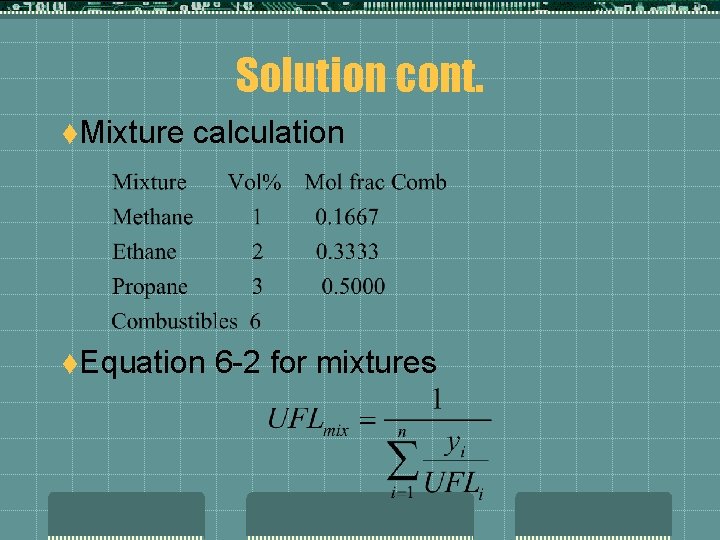
Solution cont. t. Mixture calculation t. Equation 6 -2 for mixtures

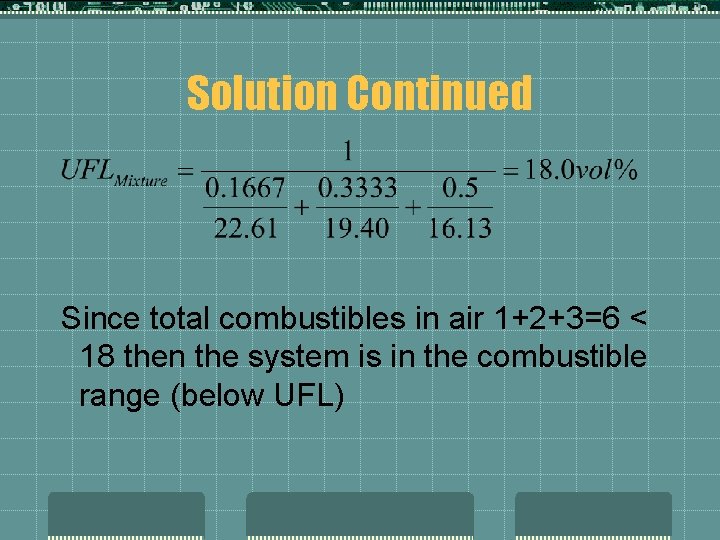
Solution Continued Since total combustibles in air 1+2+3=6 < 18 then the system is in the combustible range (below UFL)