Fire Hazards this is not an introduction to


















- Slides: 26

Fire Hazards (this is not an introduction to urban warfare)

Fire is a chemical reaction § Fire or combustion is a chemical reaction between oxygen and a combustible fluid. § The ignition point or combustion point is the temperature at which a given fuel can burst into flame.

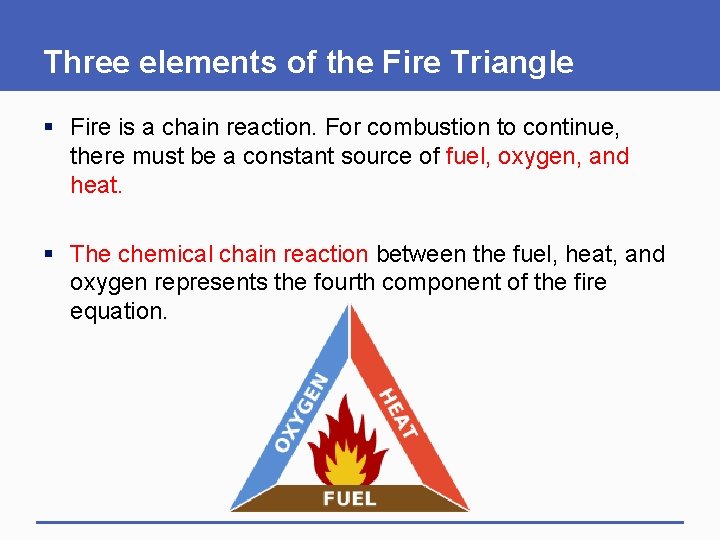
Three elements of the Fire Triangle § Fire is a chain reaction. For combustion to continue, there must be a constant source of fuel, oxygen, and heat. § The chemical chain reaction between the fuel, heat, and oxygen represents the fourth component of the fire equation.

Three elements of the Fire Triangle § Fuel: This is anything that will burn. Fuel must be available for ignition. It may be in the form of a solid, a flammable liquid or gaseous state. 1 - Solids may be wood, cloth or paper. 2 - Examples of flammable liquids are kerosene, oil and gasoline. 3 - Vapors from paint, gasoline and other flammable materials are considered gaseous.

Three elements of the Fire Triangle § Oxygen: This is needed for combustion. 21% of the air we breathe is oxygen. § Fires use and absorb this same oxygen to maintain a state of combustion (burning). § Fires also produce smoke and poisonous gases. When people breathe harmful smoke and gases, they can suffer injury.

Three elements of the Fire Triangle § Heat: Heat is needed to start a fire. For many items found in the home, the combustion temperature is 400 - 600 degrees Fahrenheit. § Source of Heat: electricity, smoking, hot surfaces and direct flame.

The 4 Stages of a Fire A fire develops typically in four stages: 1 - Incipient stage. No visible smoke, no flame and very little heat. A significant amount of invisible (but sometimes smellable) combustion particles may be created. This stage usually develops slowly. 2 - Smouldering stage. Smoke, but no flame and little heat.

The 4 Stages of a Fire 3 - Flame stage. Visible flame, more heat, often less or no smoke, particularly with flammable liquids and gas fires. 4 - Heat stage. Large amounts of heat, flame, smoke and toxic gases are produced.

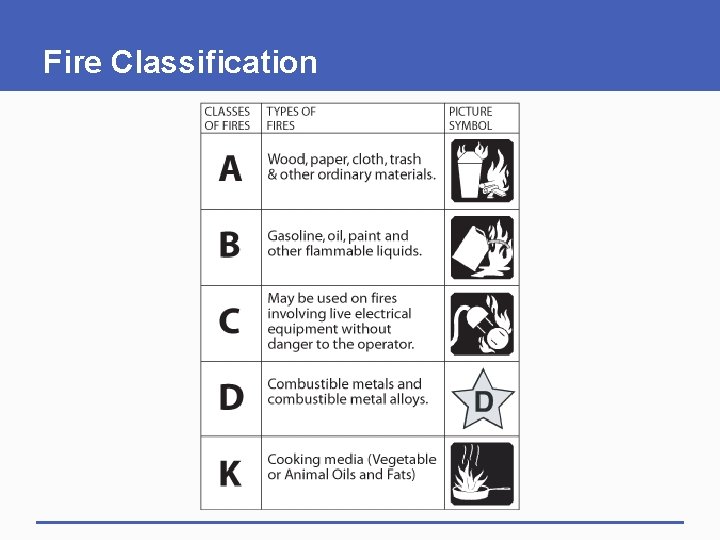
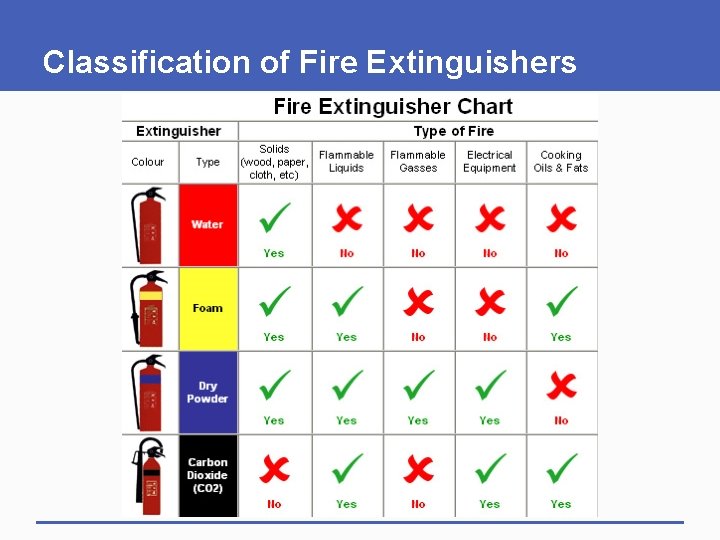
Fire Classification Fires are classified as follows: § Class A fires involve ordinary combustible materials: wood, paper, trash, plasic and cloth. § Class B fires involve flammable and combustible liquids and flammable gases such as Gasoline, Fuel oil, Paint, Butane and Propane

Fire Classification § Class C fires involve energized electrical equipment such as motors, appliances, and machinery. § Class D fires involve combustible metals such as Potassium, Sodium, Magnesium, Aluminium, Titanium, and Zirconium.

Fire Classification § Class K fires involve cooking oils and greases such as animal fats and vegetable fats.

Fire Classification

Extinguishing Agents 1 - Water: § Water is by far the most commonly used and readily available extinguishing agent. § Water works well because it has a large capacity for absorbing. Absorbing the heat cools the burning material to below its ignition temperature, thus causing the fire to go out.

Extinguishing Agents 2 - Halons: § Halogenated hydrocarbon agents, usually referred to as halons, are a group of gaseous agents that are effective in fire control. § The main disadvantage of halon is its environmental impact and cost. It is the most expensive of the extinguishing agents. Halon is also one of the chemicals connected with the depletion of the ozone layer.

Extinguishing Agents 3 - Carbon Dioxide: § Carbon dioxide (CO 2) is a gaseous fire control agent that is stored under pressure as a liquid. It is rated for class B and C fires. The major advantages of carbon dioxide are that it leaves no residue. § The main disadvantage of carbon dioxide is that it can create an oxygen deficient area where it has been used that poses a significant risk to personnel.

Extinguishing Agents 4 - Foam: § Examples of foam types include: Protein foam, Aqueous film forming foam (AFFF), Alcohol-type foam (for Polar Solvent Fires)

Extinguishing Agents 5 - Dry Powder: § Dry powder agents are designed to control fires in combustible metals (class D). § The two most common agents in this category are G-1 and Met-L-X. Dry powders function by creating a crust over the surface of the burning metal. § Graphite and sodium chloride are two common examples of these agents.

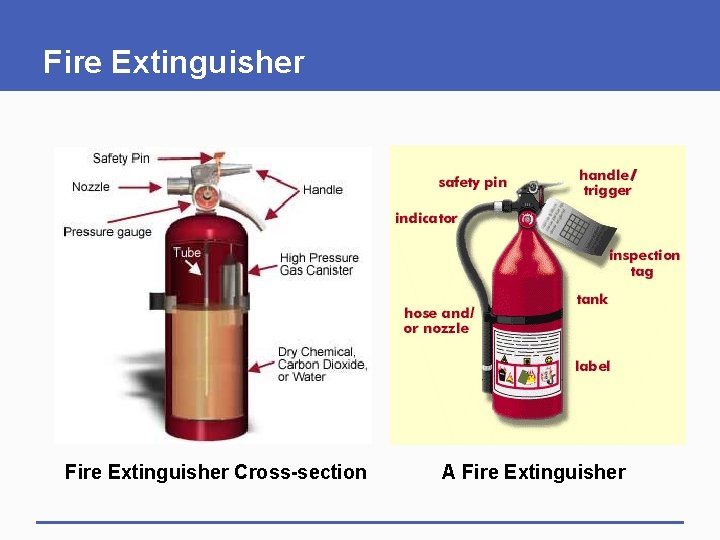
Fire Extinguisher Cross-section A Fire Extinguisher

Classification of Fire Extinguishers

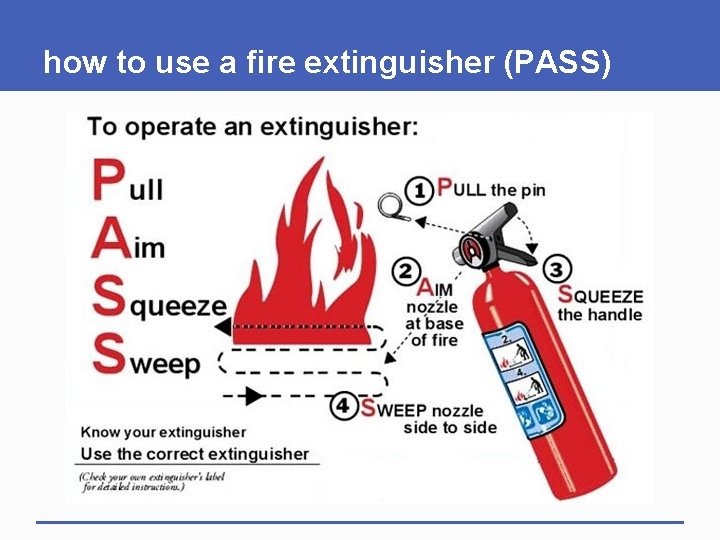
how to use a fire extinguisher (PASS) § Pull the Pin, at the top of the extinguisher. The pin releases a locking mechanism and will allow you to discharge the extinguisher. § Aim at the base of the fire, not the flames. This is important - in order to put out the fire, you must extinguish the fuel. § Squeeze the lever slowly, This will release the extinguishing agent in the extinguisher. If the handle is released, the discharge will stop. § Sweep from side to side, Using a sweeping motion, move the fire extinguisher until the fire is completely out. Be sure to read the instructions on your fire extinguisher.

how to use a fire extinguisher (PASS)

Fire Safety Procedures 1 - abide by particular fire safety procedures, such as maintaining smoke detectors and fire alarms that function properly. 2 - passageways and escape routes are not blocked or obstructed and seeing to it that emergency exits are clearly identified in the building. 3 - fire safety procedure should list items that are considered hazardous, reportable and prohibited. For instance, exposed electrical wires, flammable chemicals and personal electrical devices.

Types of sign relating to fire 1 - Prohibition signs § Circular red band a single diagonal cross bar over the activity to be banned, e. g. No Smoking. 2 - Mandatory signs § Blue circle with pictogram or text, as in 'Fire Door Keep Shut'.

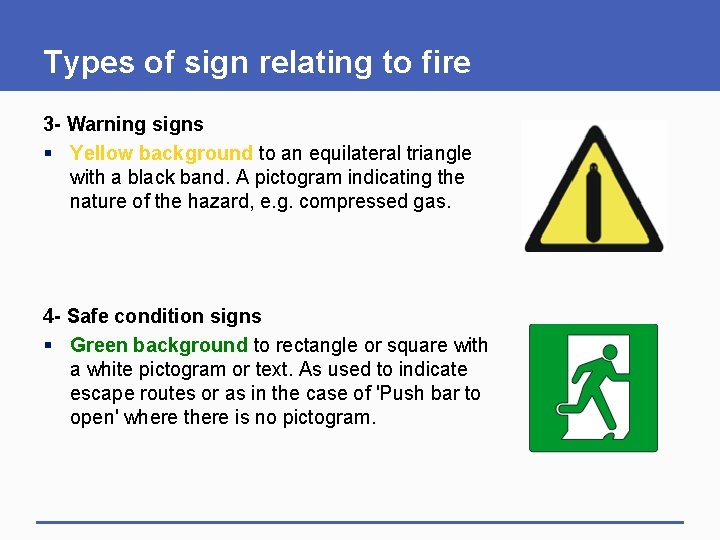
Types of sign relating to fire 3 - Warning signs § Yellow background to an equilateral triangle with a black band. A pictogram indicating the nature of the hazard, e. g. compressed gas. 4 - Safe condition signs § Green background to rectangle or square with a white pictogram or text. As used to indicate escape routes or as in the case of 'Push bar to open' where there is no pictogram.

Types of sign relating to fire 5 - Fire fighting § Red rectangle or square with a white pictogram indicating the meaning e. g. location of fire fighting equipment

§ Alkali metals in water, accurate! - You. Tube