Finish spectrum visible light energy of EM waves

Finish spectrum, visible light, energy of EM waves
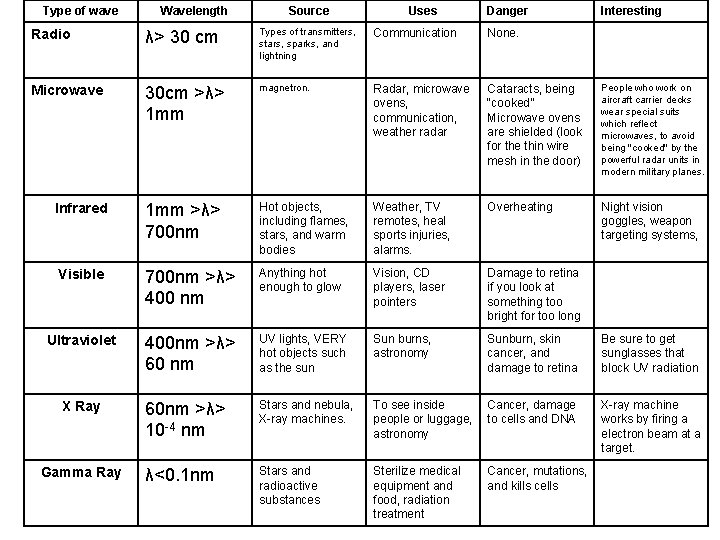
Type of wave Wavelength Source Uses Danger Interesting Radio λ> 30 cm Types of transmitters, stars, sparks, and lightning Communication None. Microwave 30 cm >λ> 1 mm magnetron. Radar, microwave ovens, communication, weather radar Cataracts, being “cooked” Microwave ovens are shielded (look for the thin wire mesh in the door) People who work on aircraft carrier decks wear special suits which reflect microwaves, to avoid being "cooked" by the powerful radar units in modern military planes. Infrared 1 mm >λ> 700 nm Hot objects, including flames, stars, and warm bodies Weather, TV remotes, heal sports injuries, alarms. Overheating Night vision goggles, weapon targeting systems, Visible 700 nm >λ> 400 nm Anything hot enough to glow Vision, CD players, laser pointers Damage to retina if you look at something too bright for too long Ultraviolet 400 nm >λ> 60 nm UV lights, VERY hot objects such as the sun Sun burns, astronomy Sunburn, skin cancer, and damage to retina Be sure to get sunglasses that block UV radiation 60 nm >λ> 10 -4 nm Stars and nebula, X-ray machines. To see inside people or luggage, astronomy Cancer, damage to cells and DNA X-ray machine works by firing a electron beam at a target. λ<0. 1 nm Stars and radioactive substances Sterilize medical equipment and food, radiation treatment Cancer, mutations, and kills cells X Ray Gamma Ray
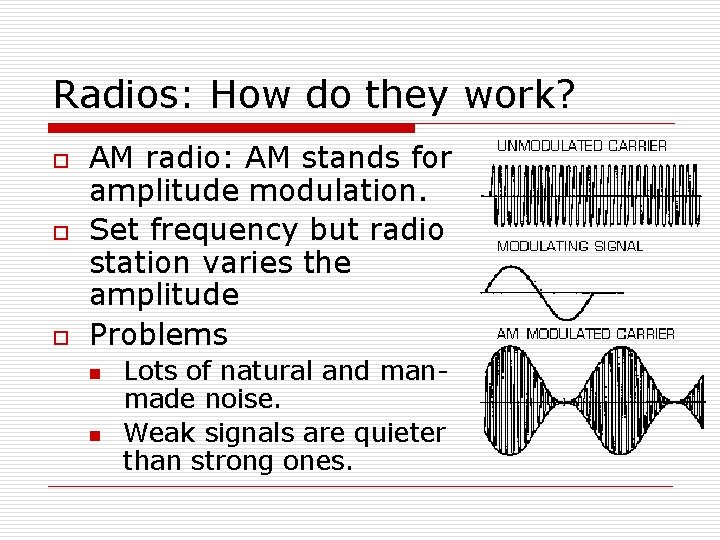
Radios: How do they work? o o o AM radio: AM stands for amplitude modulation. Set frequency but radio station varies the amplitude Problems n n Lots of natural and manmade noise. Weak signals are quieter than strong ones.
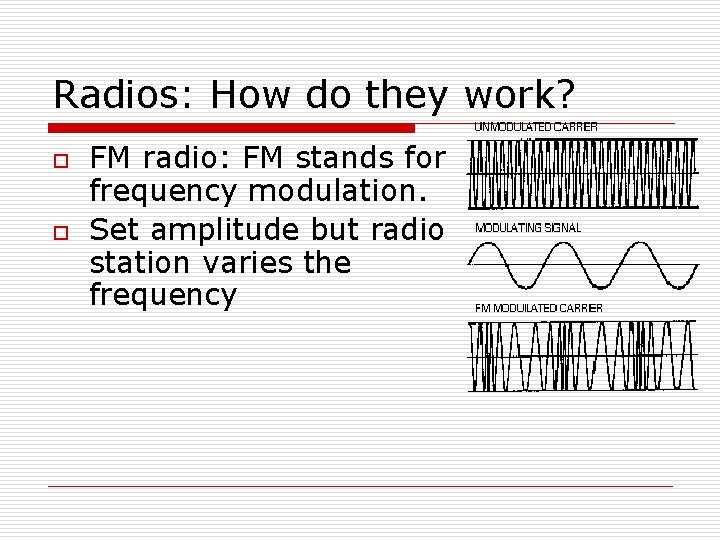
Radios: How do they work? o o FM radio: FM stands for frequency modulation. Set amplitude but radio station varies the frequency

Microwave ovens o Water. There are trillions of water molecules in a single drop. The temperature of the water depends on the motion of the molecules. They move with respect to each other and vibrate. n The hotter the water, the more activity.

Microwaves continued o o Water molecules absorb microwaves at a certain frequency. This extra energy causes an increased amount of vibrations in the molecules. As the water molecules vibrate, they bump into other molecules, transferring energy or heat.

Visible Light o A few things you need to know: n n o For Violet light, λ= 400 nm For red light, λ= 700 nm Example: Compare the frequency of red light compared to violet light.

Visible Light o c=fλ Plug in the numbers n n n o o o Red λ= 700 nm = 700*10 -9 m Violet λ= 400 nm = 400*10 -9 m C=3. 00*108 m/s Frequency of Red light=4. 3*1014 Hz Frequency of Violet light=7. 5*1014 Hz Notice the trend, Violet has a higher frequency than Red
Light Particle o o Photon—A discrete unit of light energy A photon is “localized energy”
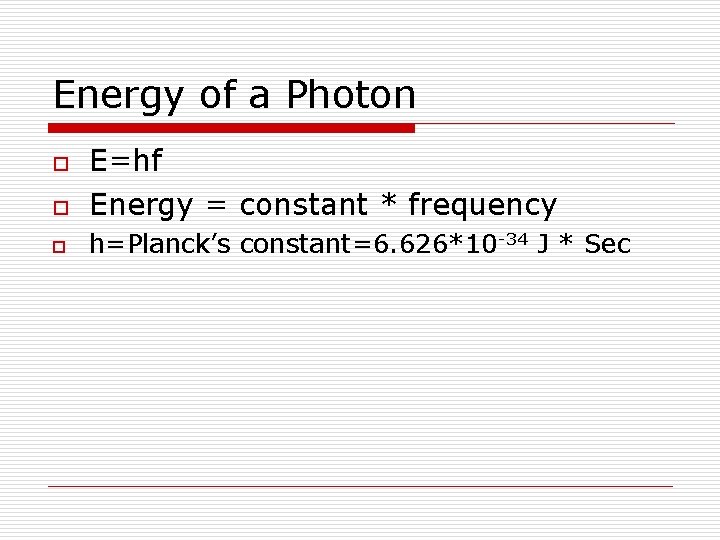
Energy of a Photon o E=hf Energy = constant * frequency o h=Planck’s constant=6. 626*10 -34 J * Sec o
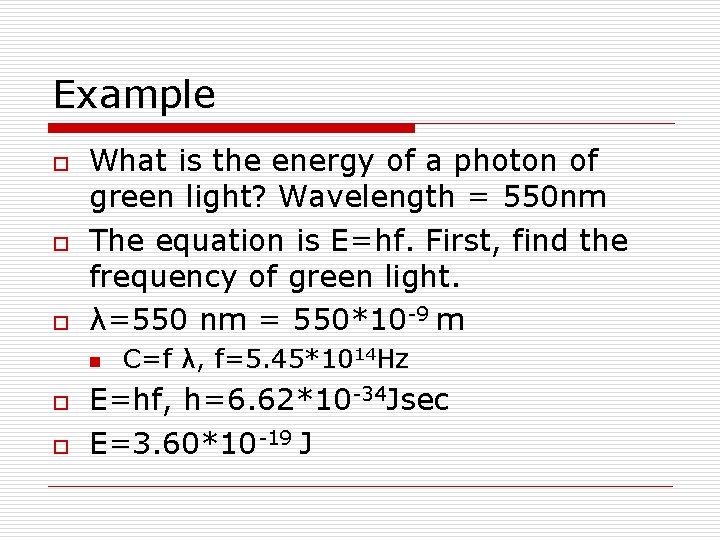
Example o o o What is the energy of a photon of green light? Wavelength = 550 nm The equation is E=hf. First, find the frequency of green light. λ=550 nm = 550*10 -9 m n o o C=f λ, f=5. 45*1014 Hz E=hf, h=6. 62*10 -34 Jsec E=3. 60*10 -19 J

Example 2 o o o If the energy of a photon is 2. 5*10 -18 J, what is the frequency of that photon? What is the wavelength? Answer: Frequency =3. 77*1015 Hz Wavelength = 7. 944*10 -8 m
- Slides: 12