Finding Volume The Water Displacement Method Mr Johns






















- Slides: 35

Finding Volume The Water Displacement Method Mr. Johns February 22, 2016

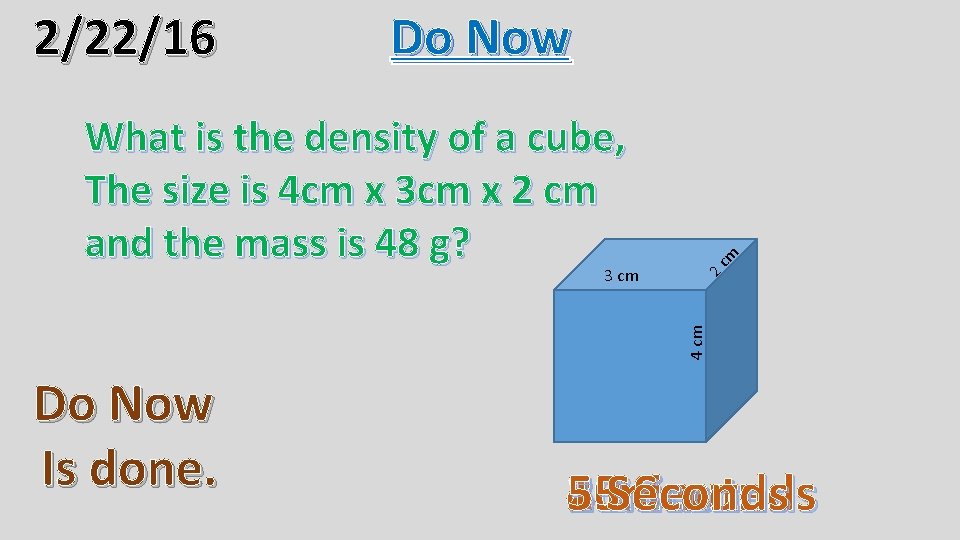
What is the density of a cube, The size is 4 cm x 3 cm x 2 cm and the mass is 48 g? 4 cm 3 cm cm Do Now 2 2/22/16 Do Now Is done. 1 4 3 2 5 30 minute 15 minutes Seconds

2/22/16 Objective SWBAT explain that materials have characteristic densities because of the different mass, size and arrangement of their atoms. Students will be able to use the volume displacement method to find the volume of an object. 1 2 5 30 minute 15 minutes Seconds

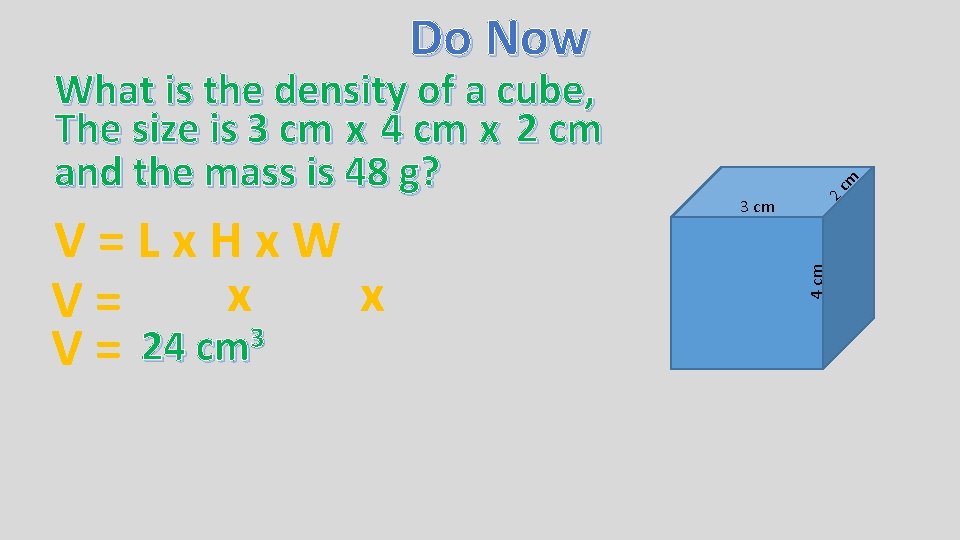
V=Lx. Hx. W x x V= 3 24 cm V= 2 3 cm 4 cm What is the density of a cube, The size is 3 cm x 4 cm x 2 cm and the mass is 48 g? cm Do Now

Find the Volume 3 1) 2 cm x 3 cm x 1 cm = 6 cm 3 2) 4 cm x 3 cm x 2 cm = 24 2) 4 cm x 3 cm x 2 cm = cm 3 3) 2 cm x 2 cm = 8 cm 3 4) 10 cm x 2 cm = 40 4) 10 cm x 2 cm = cm 3 5) 12 cm x 10 cm x 2 cm = 240 cm V = L x W x H V = 2 cm x 3 cm x 1 cm 1 4 3 2 5 30 15 minutes Seconds 3 V = 6 cm Question 1 – 4 Answered in this format

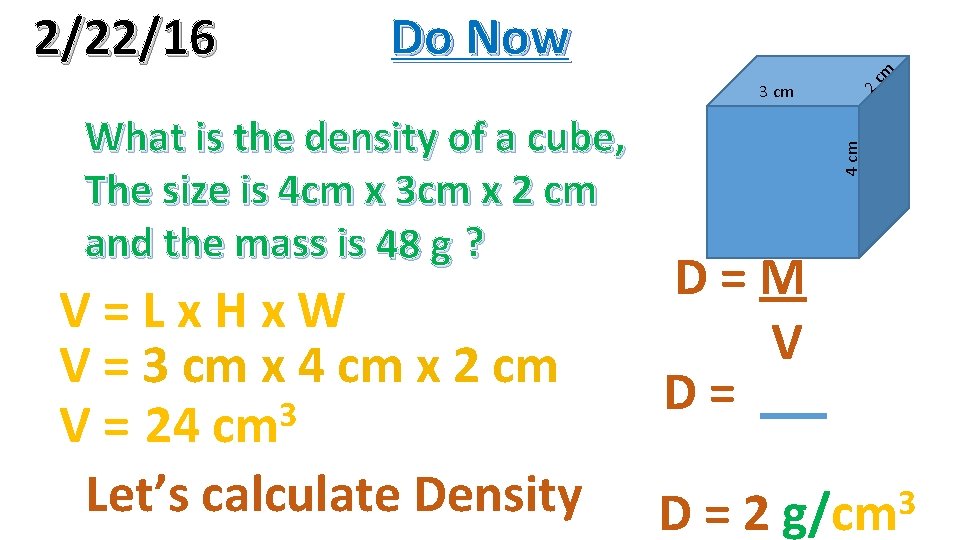
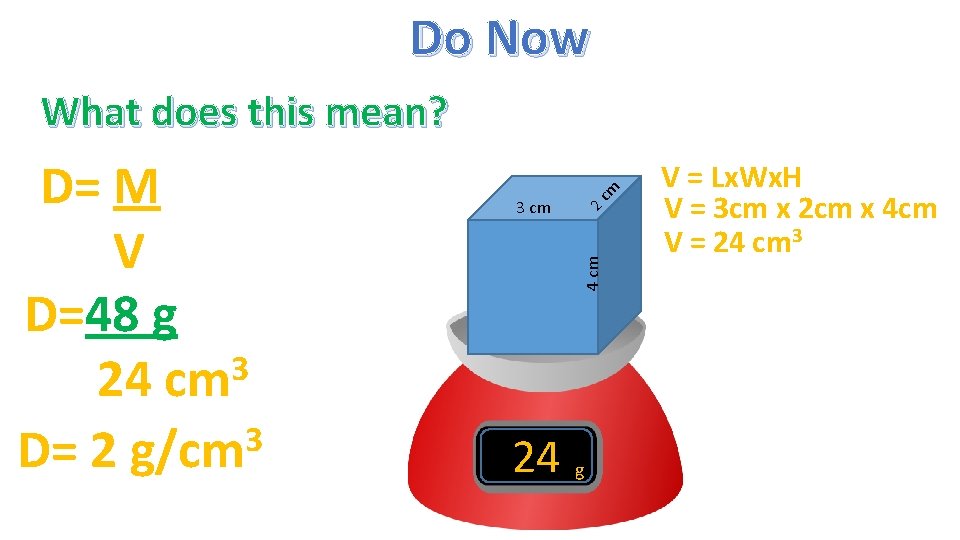
What is the density of a cube, The size is 4 cm x 3 cm x 2 cm and the mass is 48 g ? V=Lx. Hx. W V = 3 cm x 4 cm x 2 cm 3 V = 24 cm Let’s calculate Density 4 cm 3 cm cm Do Now 2 2/22/16 D=M V D= D=2 3 g/cm

Find the Volume 3 1) 2 cm x 3 cm x 1 cm = 6 cm 3 2) 4 cm x 3 cm x 2 cm = 24 2) 4 cm x 3 cm x 2 cm = cm 3 3) 2 cm x 2 cm = 8 cm 3 4) 10 cm x 2 cm = 40 4) 10 cm x 2 cm = cm 3 5) 12 cm x 10 cm x 2 cm = 240 cm

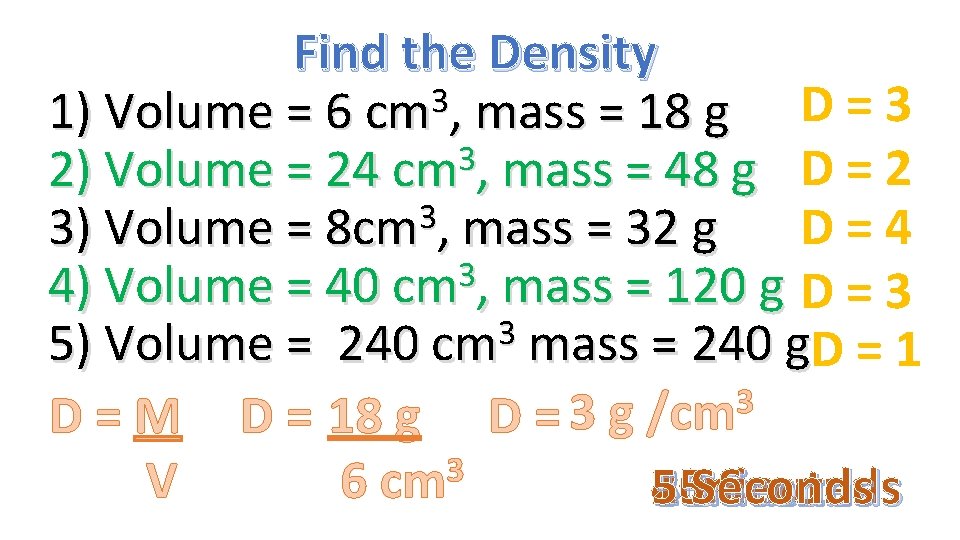
Find the Density 3 1) Volume = 6 cm , mass = 18 g D = 3 3 2) Volume = 24 cm , mass = 48 g D = 2 3 3) Volume = 8 cm , mass = 32 g D = 4 3 4) Volume = 40 cm , mass = 120 g D = 3 3 5) Volume = 240 cm mass = 240 g. D = 1 3 D = M D = 18 g D = 3 g /cm 3 6 cm V 1 4 3 2 5 30 minute 15 minutes Seconds

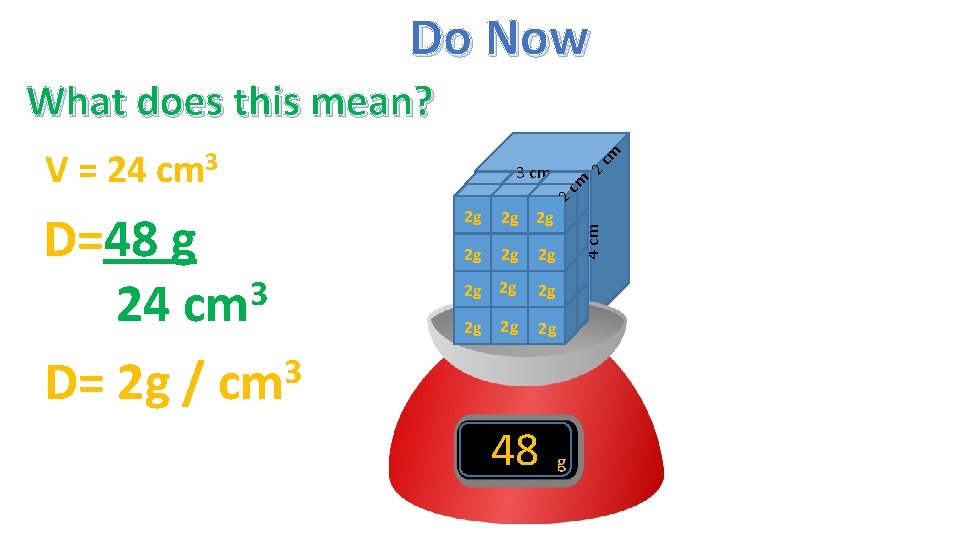
Do Now 2 3 cm 4 cm D= M V D=48 g 3 24 cm 3 D= 2 g/cm cm What does this mean? 24 g V = Lx. Wx. H V = 3 cm x 2 cm x 4 cm V = 24 cm 3

Do Now 2 g 2 g 2 g cm 2 g 2 g 1 1 cm 2 g 1 cm D=48 g 3 24 cm 3 D= 2 g / cm 2 g 2 g 2 4 cm 2 3 cm cm V = 24 cm 3 cm What does this mean? 2 g 48 g

Vocabulary 8. Water displacement 9. Diameter 10. Milliliter vs. millimeter 11. Graduated Cylinder


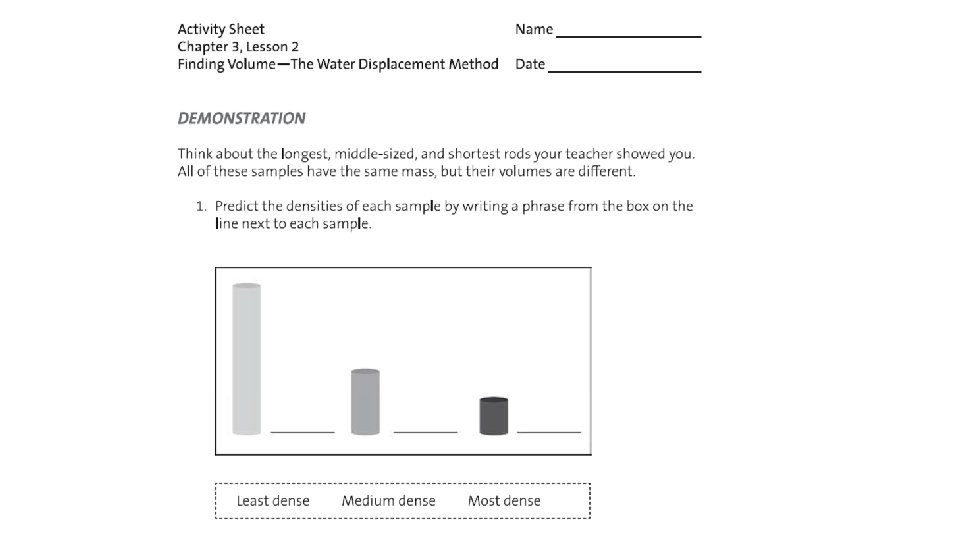
2. Explain why you think each rod is either the most, medium or least dense?


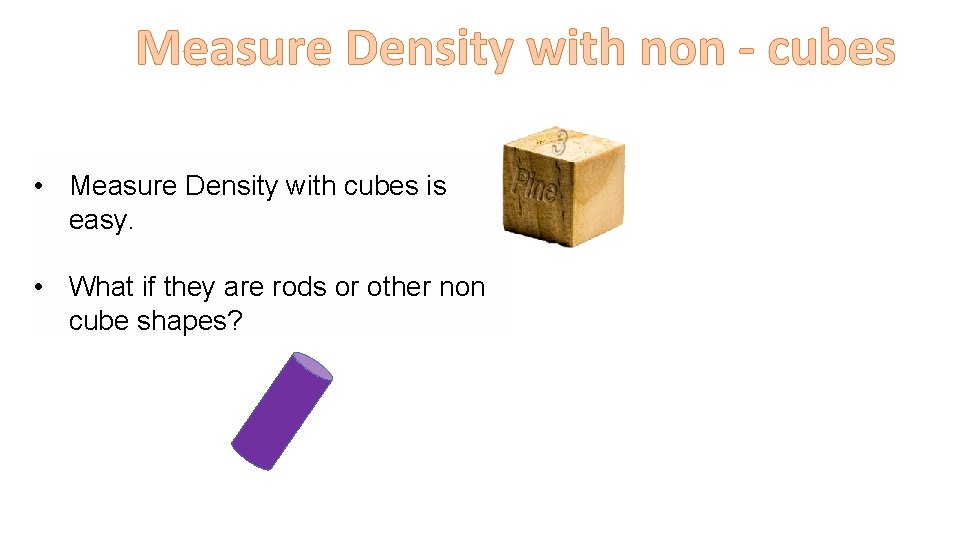
Measure Density with non - cubes • Measure Density with cubes is easy. • What if they are rods or other non cube shapes?

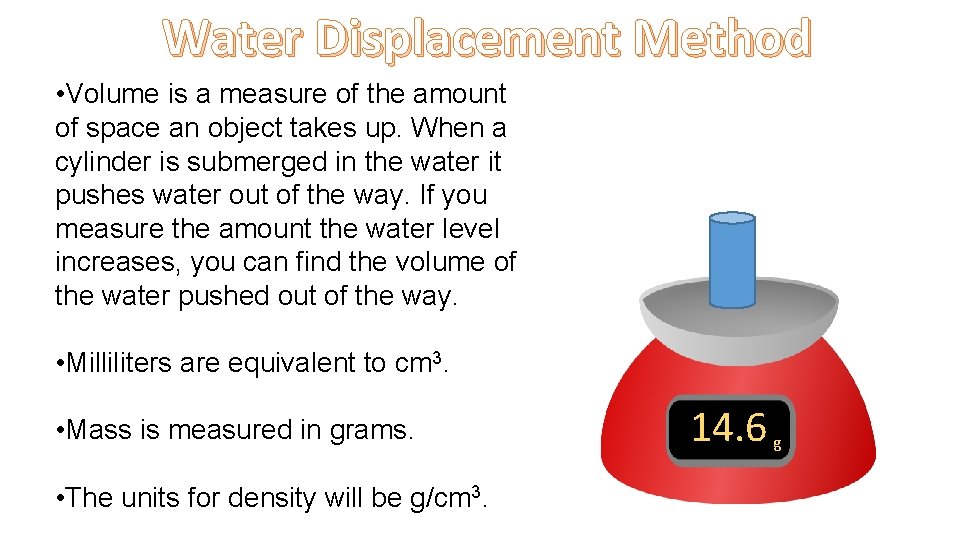
Water Displacement Method • Volume is a measure of the amount of space an object takes up. When a cylinder is submerged in the water it pushes water out of the way. If you measure the amount the water level increases, you can find the volume of the water pushed out of the way. • Milliliters are equivalent to cm 3. • Mass is measured in grams. • The units for density will be g/cm 3. 14. 6 g

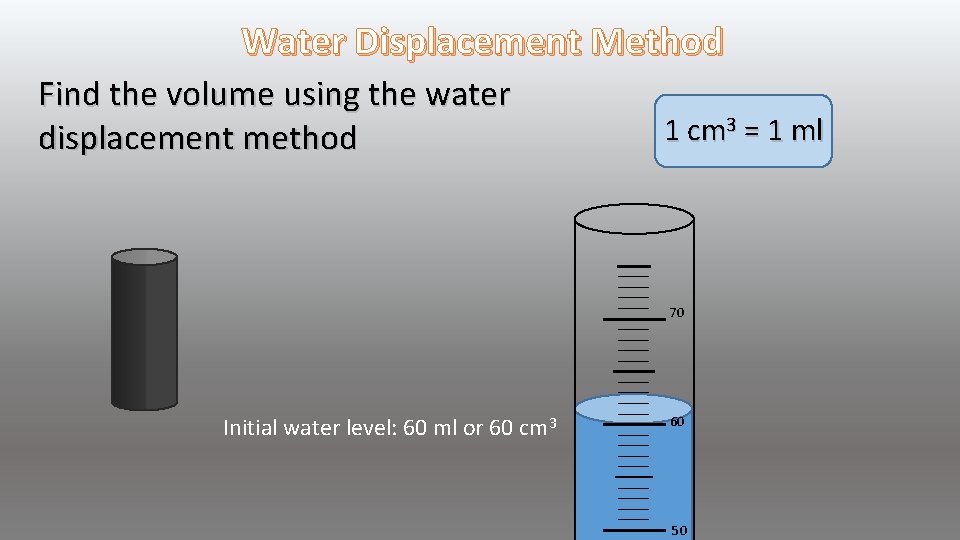
Water Displacement Method Find the volume using the water displacement method 1 cm 3 = 1 ml 70 Initial water level: 60 ml or 60 cm 3 60 50

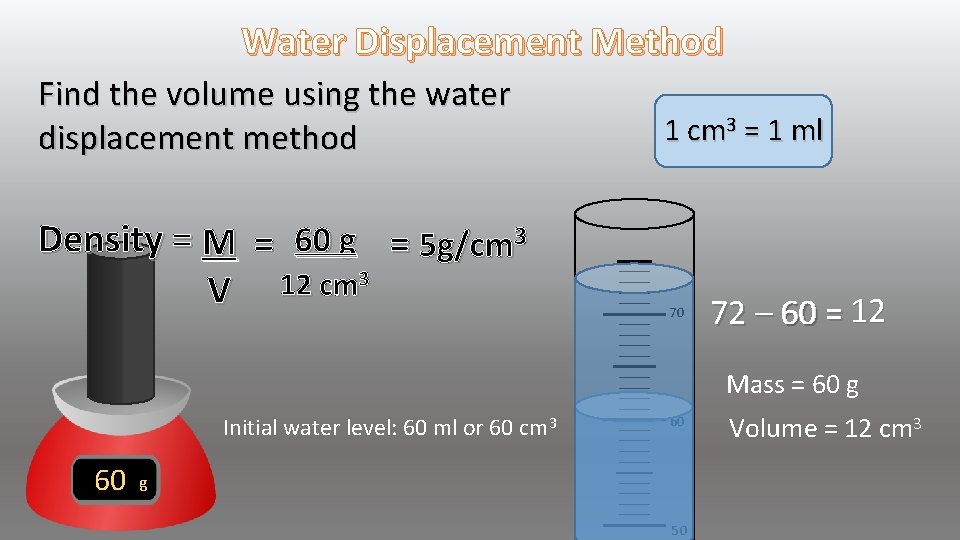
Water Displacement Method Find the Mass = 60 g 0 60 g

Water Displacement Method Find the volume using the water displacement method Density = M = 60 g = 5 g/cm 3 3 12 cm V 1 cm 3 = 1 ml 70 60 12 72 – 60 = Mass = 60 g Initial water level: 60 ml or 60 cm 3 60 60 g 50 Volume = 12 cm 3

Question: Can you use density to identify different materials?


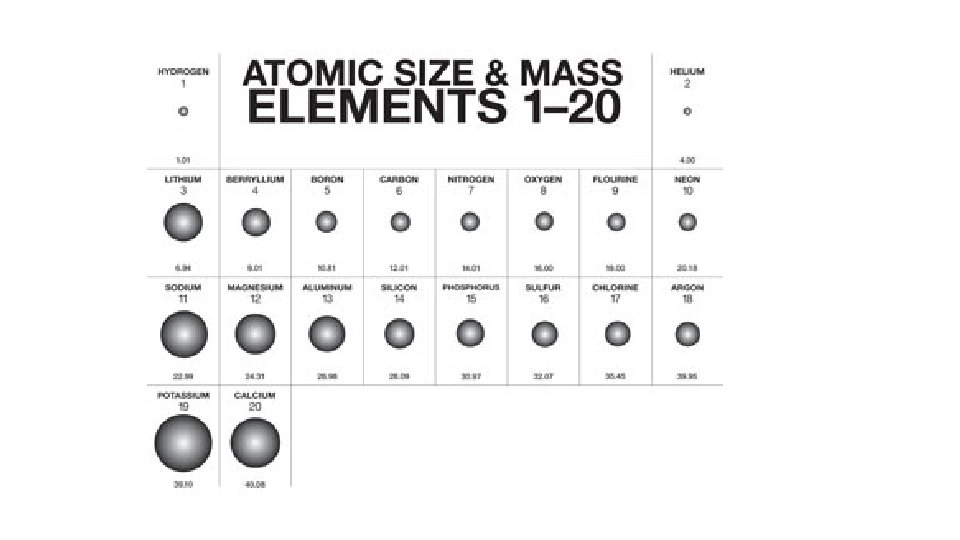
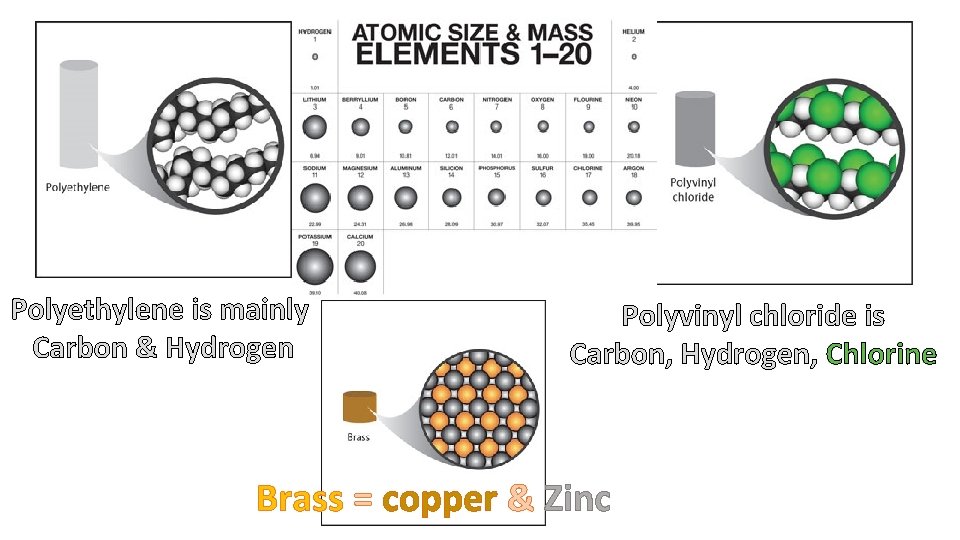
Polyethylene is mainly Carbon & Hydrogen Polyvinyl chloride is Carbon, Hydrogen, Chlorine Brass = copper & Zinc












