Finding the Amount of Excess Reactant Left Over
- Slides: 4

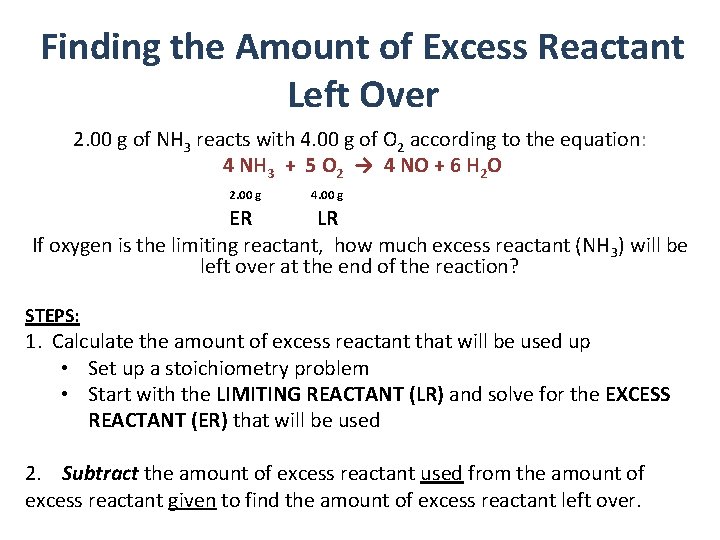
Finding the Amount of Excess Reactant Left Over 2. 00 g of NH 3 reacts with 4. 00 g of O 2 according to the equation: 4 NH 3 + 5 O 2 → 4 NO + 6 H 2 O 2. 00 g 4. 00 g ER LR If oxygen is the limiting reactant, how much excess reactant (NH 3) will be left over at the end of the reaction? STEPS: 1. Calculate the amount of excess reactant that will be used up • Set up a stoichiometry problem • Start with the LIMITING REACTANT (LR) and solve for the EXCESS REACTANT (ER) that will be used 2. Subtract the amount of excess reactant used from the amount of excess reactant given to find the amount of excess reactant left over.

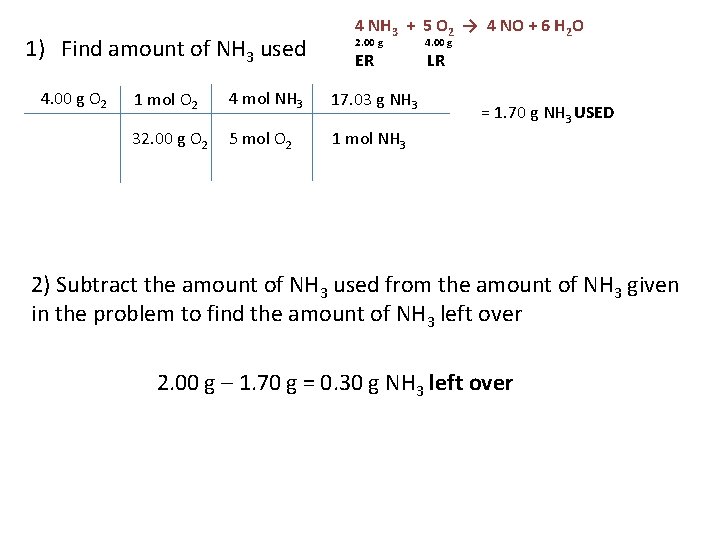
1) Find amount of NH 3 used 4. 00 g O 2 4 NH 3 + 5 O 2 → 4 NO + 6 H 2 O 2. 00 g ER 1 mol O 2 4 mol NH 3 17. 03 g NH 3 32. 00 g O 2 5 mol O 2 1 mol NH 3 4. 00 g LR = 1. 70 g NH 3 USED 2) Subtract the amount of NH 3 used from the amount of NH 3 given in the problem to find the amount of NH 3 left over 2. 00 g – 1. 70 g = 0. 30 g NH 3 left over

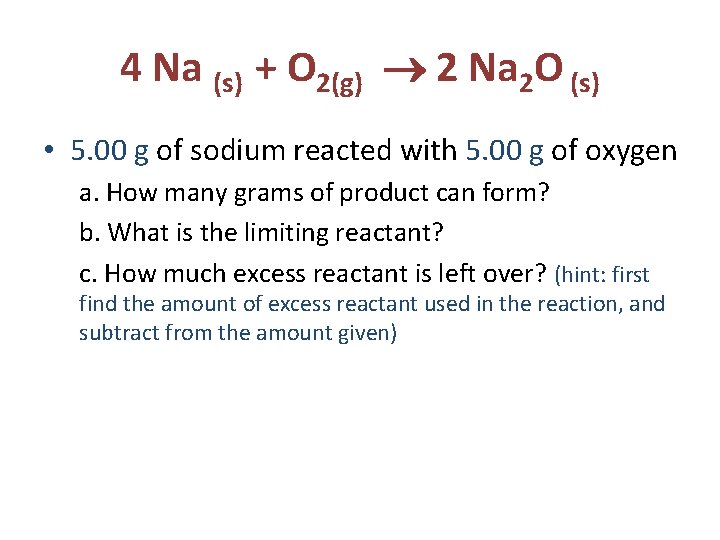
4 Na (s) + O 2(g) 2 Na 2 O (s) • 5. 00 g of sodium reacted with 5. 00 g of oxygen a. How many grams of product can form? b. What is the limiting reactant? c. How much excess reactant is left over? (hint: first find the amount of excess reactant used in the reaction, and subtract from the amount given)

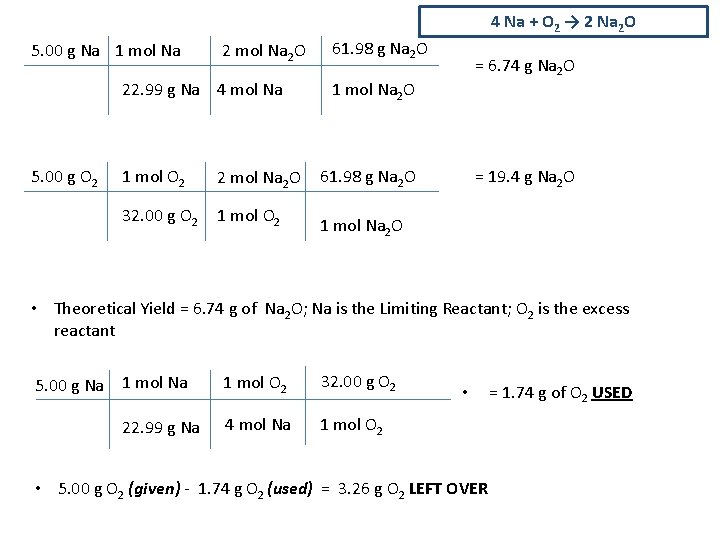
4 Na + O 2 → 2 Na 2 O 5. 00 g Na 1 mol Na 2 O 22. 99 g Na 4 mol Na 5. 00 g O 2 1 mol O 2 2 mol Na 2 O 32. 00 g O 2 1 mol O 2 61. 98 g Na 2 O = 6. 74 g Na 2 O 1 mol Na 2 O 61. 98 g Na 2 O = 19. 4 g Na 2 O 1 mol Na 2 O • Theoretical Yield = 6. 74 g of Na 2 O; Na is the Limiting Reactant; O 2 is the excess reactant 5. 00 g Na 1 mol O 2 32. 00 g O 2 22. 99 g Na 4 mol Na 1 mol O 2 • • 5. 00 g O 2 (given) - 1. 74 g O 2 (used) = 3. 26 g O 2 LEFT OVER = 1. 74 g of O 2 USED