Final Review Chp 8 and 9 Vocabulary Covalent

Final Review: Chp. 8 and 9
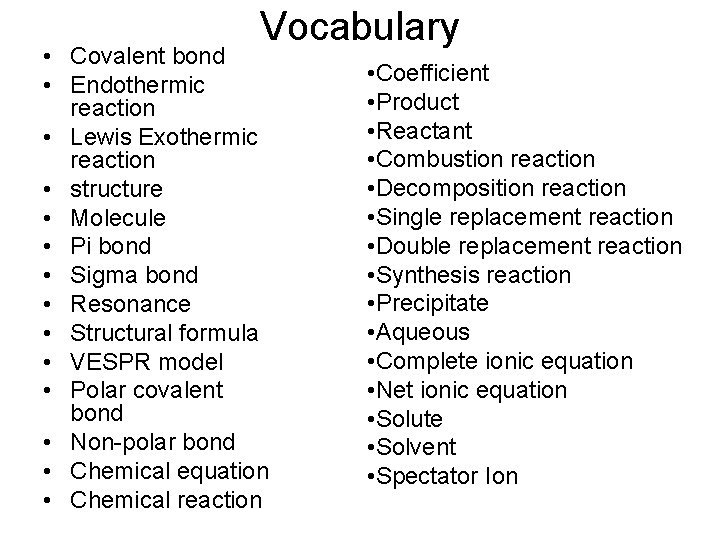
Vocabulary • Covalent bond • Endothermic reaction • Lewis Exothermic reaction • structure • Molecule • Pi bond • Sigma bond • Resonance • Structural formula • VESPR model • Polar covalent bond • Non-polar bond • Chemical equation • Chemical reaction • Coefficient • Product • Reactant • Combustion reaction • Decomposition reaction • Single replacement reaction • Double replacement reaction • Synthesis reaction • Precipitate • Aqueous • Complete ionic equation • Net ionic equation • Solute • Solvent • Spectator Ion
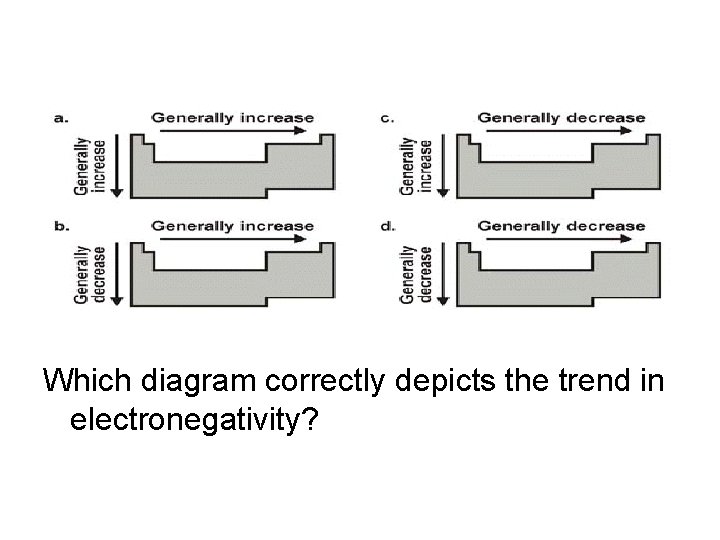
Which diagram correctly depicts the trend in electronegativity?
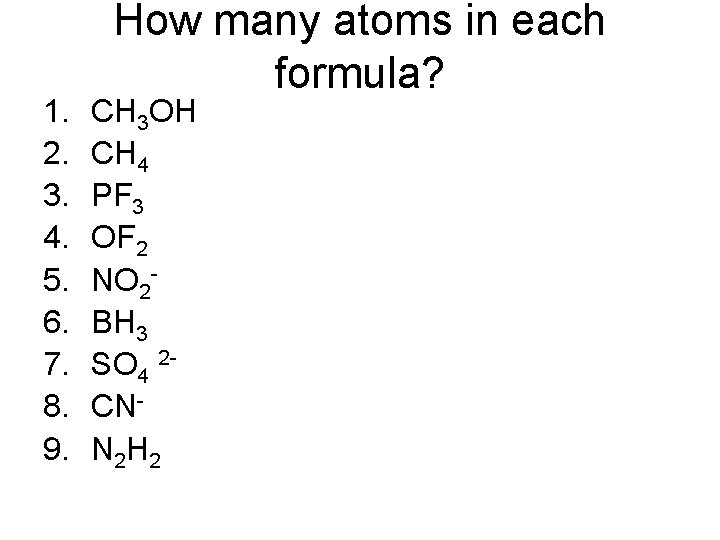
1. 2. 3. 4. 5. 6. 7. 8. 9. How many atoms in each formula? CH 3 OH CH 4 PF 3 OF 2 NO 2 BH 3 SO 4 2 CNN 2 H 2

Covalent Bonds • How many covalent bonds can elements in the following groups form: – Group 1 (alkali metals) – Group 2 (alkali earth metals) – Group 3 – Group 4 – Group 5 – Group 6 – Group 7 (halogens) – Group 8 ( noble gases)
Diatomic Molecules • List the 7 diatomic molecules:
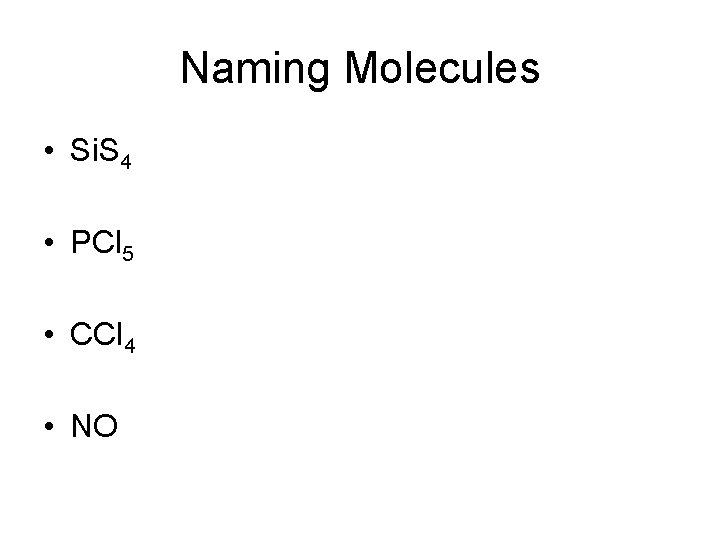
Naming Molecules • Si. S 4 • PCl 5 • CCl 4 • NO

Writing Formulas • Sulfur difluoride • Silicon tetrachloride • Chlorine trifluoride • Tetrasulfur heptanitride
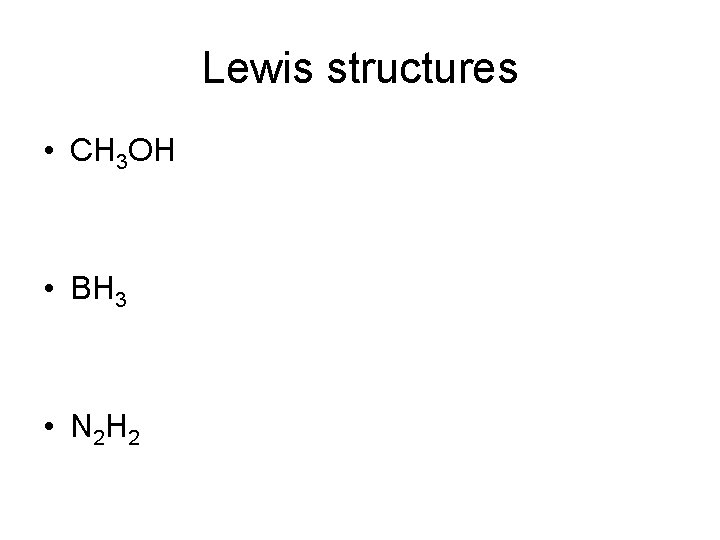
Lewis structures • CH 3 OH • BH 3 • N 2 H 2
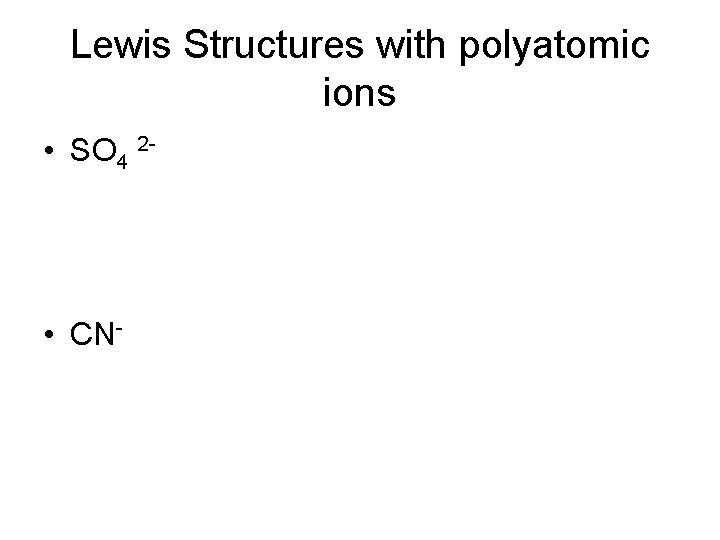
Lewis Structures with polyatomic ions • SO 4 2 - • CN-
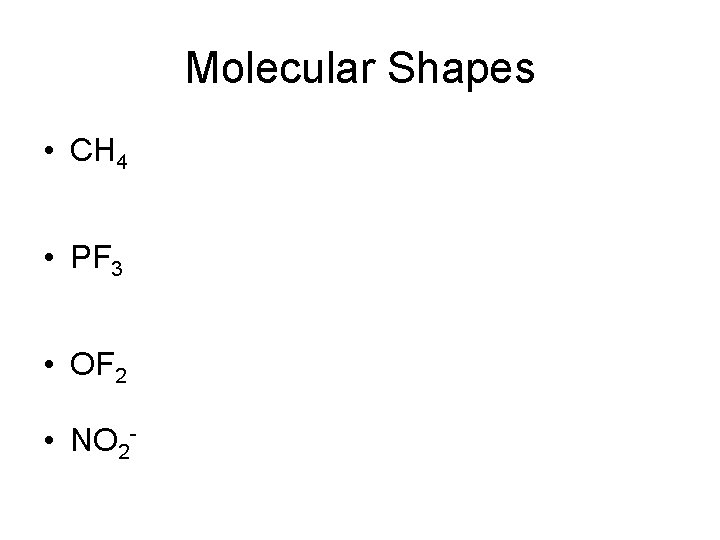
Molecular Shapes • CH 4 • PF 3 • OF 2 • NO 2 -
Lewis Structures with resonance • NO 3– • CO 32 -

Label the following as reactants or products • Zinc and lead (II) nitrate react to form zinc nitrate and lead. • 2 SO 2 + O 2 2 AL(OH)3 + 3 Ca. SO 4 • 2 Be + O 2 2 Be. O • 2 Pb. O 2 2 Pb. O + O 2

List the following GENERAL equations • Combustion • Synthesis • Decomposition • Double replacement • Single replacement
Name the type of chemical reaction • 2 SO 2 + 3 O 2 2 SO 4 • 2 Be + O 2 2 Be. O • 2 Pb. O 2 2 Pb. O + O 2 • C 2 H 6 + O 2 CO 2 + H 2 O • Li + Na. OH Li. OH + Na
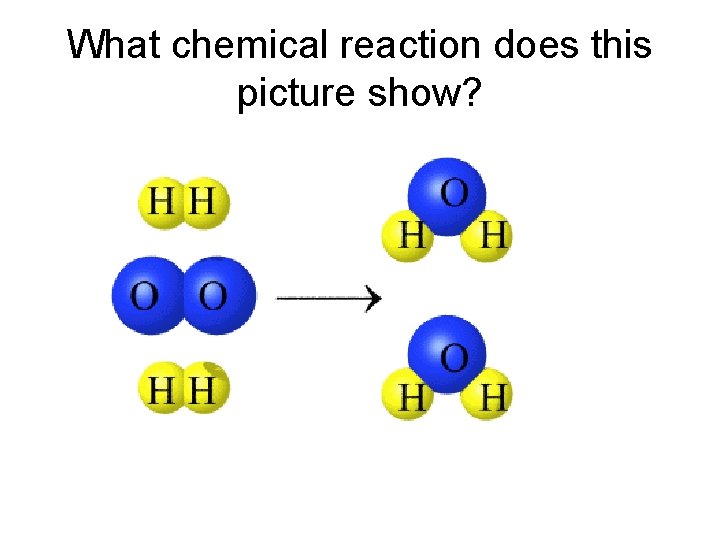
What chemical reaction does this picture show?
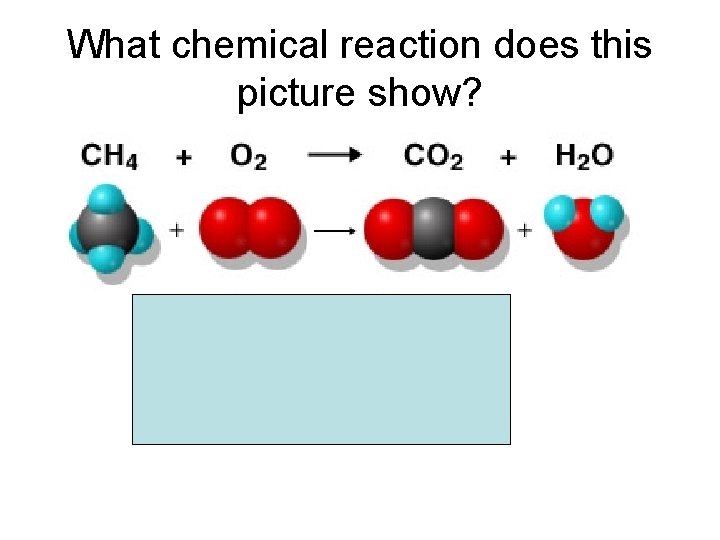
What chemical reaction does this picture show?
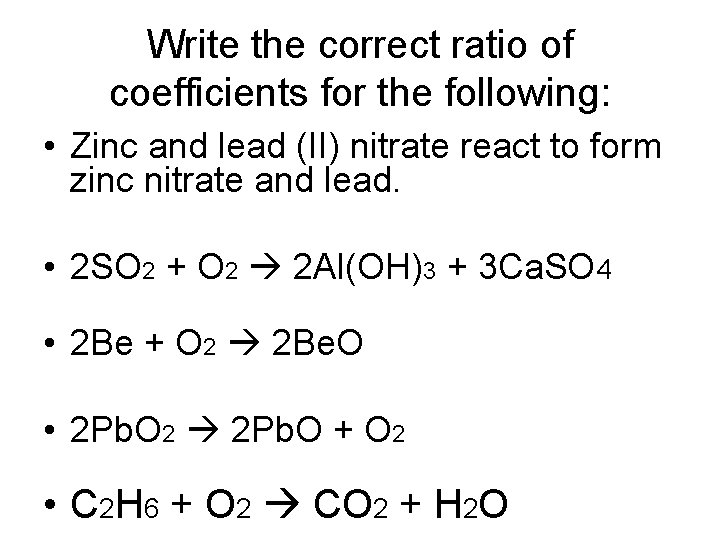
Write the correct ratio of coefficients for the following: • Zinc and lead (II) nitrate react to form zinc nitrate and lead. • 2 SO 2 + O 2 2 Al(OH)3 + 3 Ca. SO 4 • 2 Be + O 2 2 Be. O • 2 Pb. O 2 2 Pb. O + O 2 • C 2 H 6 + O 2 CO 2 + H 2 O
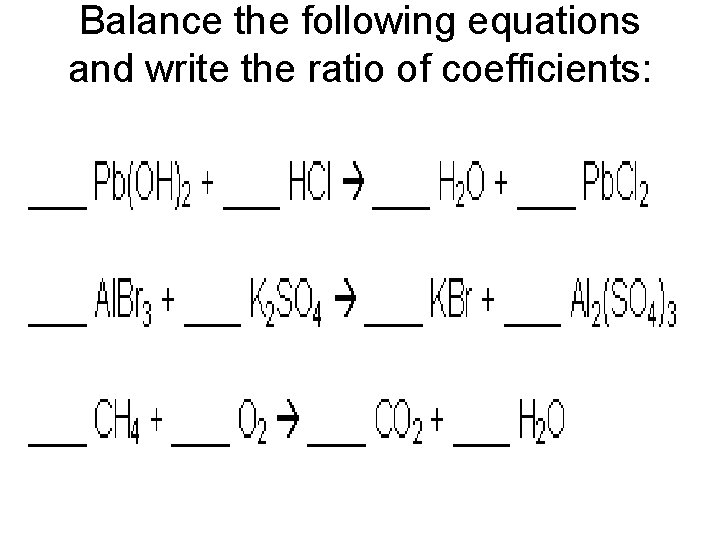
Balance the following equations and write the ratio of coefficients:
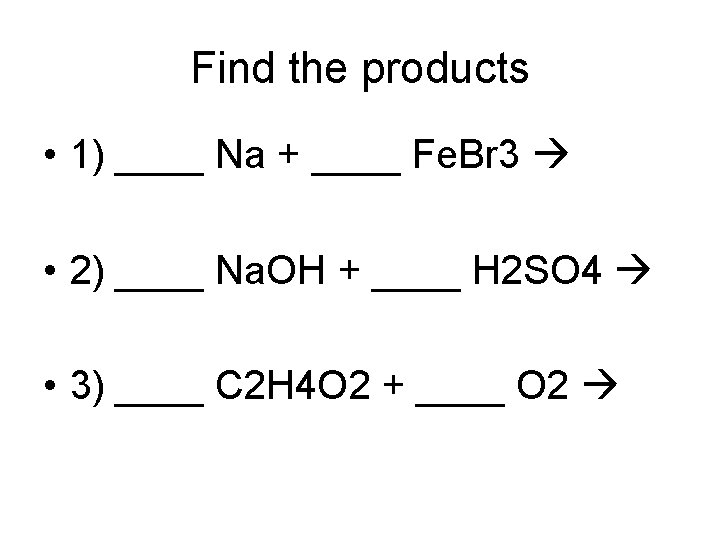
Find the products • 1) ____ Na + ____ Fe. Br 3 • 2) ____ Na. OH + ____ H 2 SO 4 • 3) ____ C 2 H 4 O 2 + ____ O 2

Write the total ionic equation

Write the net ionic equation
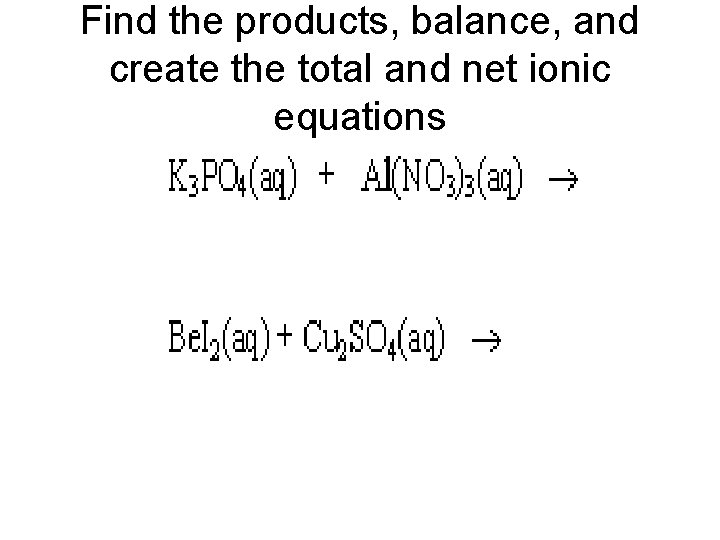
Find the products, balance, and create the total and net ionic equations
- Slides: 23