Final Atomic Scientists Nuclear Particles Potpourri Model Chem










































- Slides: 53

Final Atomic Scientists Nuclear Particles Potpourri Model Chem 100 100 100 200 200 200 300 300 300 400 400 400 500 500 500

100 Atomic Model Name the apparatus used to discover the electron Answer

Atomic Model 100 Cathode ray tube

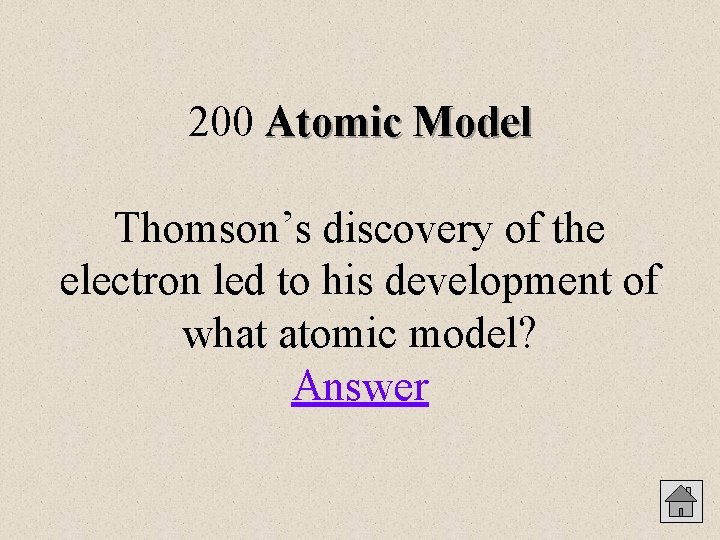
200 Atomic Model Thomson’s discovery of the electron led to his development of what atomic model? Answer

Atomic Model 200 Plum Pudding Model

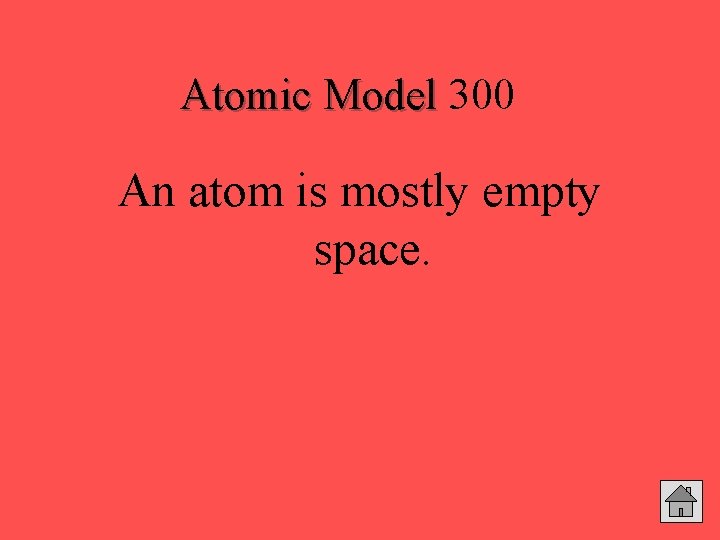
300 Atomic Model What conclusion can be drawn from the fact that most of the alpha particles passed straight through the foil in the Gold Foil Experiment? Answer

Atomic Model 300 An atom is mostly empty space.

400 Atomic Model What conclusion can be drawn from the fact that some of the alpha particles were deflected by (bounced back from) the foil in the Gold Foil Experiment? Answer

Atomic Model 400 Answer Atoms have a small, dense, positive nucleus.

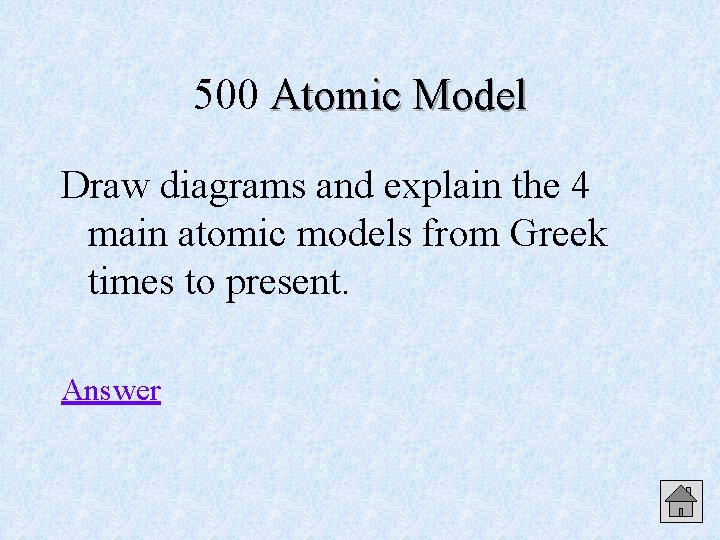
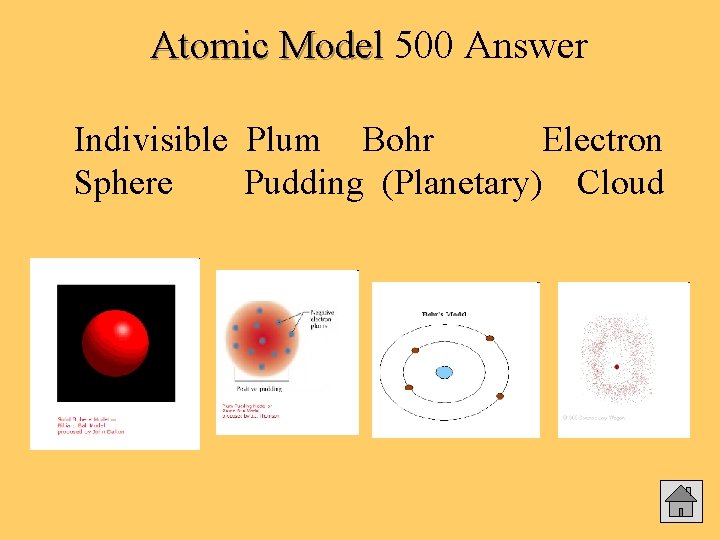
500 Atomic Model Draw diagrams and explain the 4 main atomic models from Greek times to present. Answer

Atomic Model 500 Answer Indivisible Plum Bohr Electron Sphere Pudding (Planetary) Cloud

100 Scientists Name the scientist who used the Oil Drop Experiment to determine the charge on an electron. Answer

Scientists 100 Answer Millikan

Scientists 200 Answer • Discovered and named two new elements. • First woman to earn a Nobel Prize. • First person to win 2 Nobels. • Established use of X-Rays in WWI.

200 Scientists • Describe two of Marie Curie’s unique accomplishments as a scientist. Answer

300 Scientists Name the discoverer of the neutron. Answer

Scientists 300 James Chadwick

400 Scientists Describe two corrections to Dalton’s 1808 Atomic Theory. Answer

Scientists 400 Answer • Atoms are divisible (they are made of smaller particles) • Atoms of same element can have different masses (isotopes) • Atoms can be created and destroyed (nuclear chemistry)

500 Scientists Name the scientists credited with the discovery of radioactivity. Explain how this discovery changed the idea of the “indivisible” atom. Answer

Scientists 500 Answer Henri Becquerel, Marie Curie, Pierre Curie The fact that something can be emitted from the atom indicated that it must have subatomic particles.

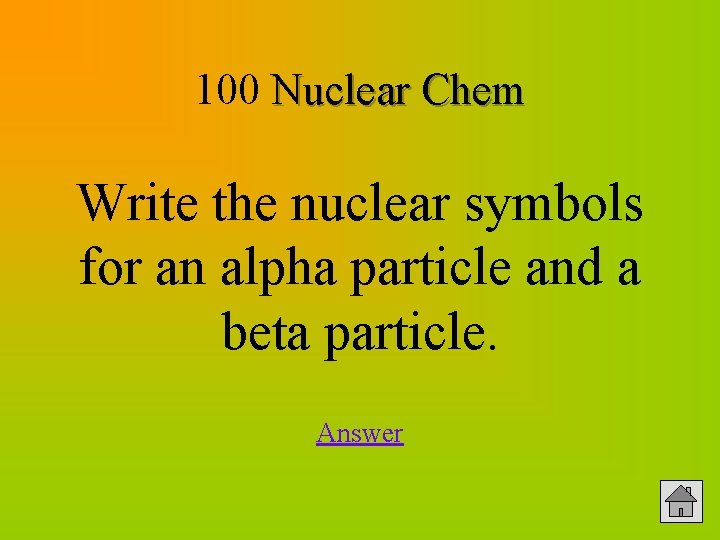
100 Nuclear Chem Write the nuclear symbols for an alpha particle and a beta particle. Answer

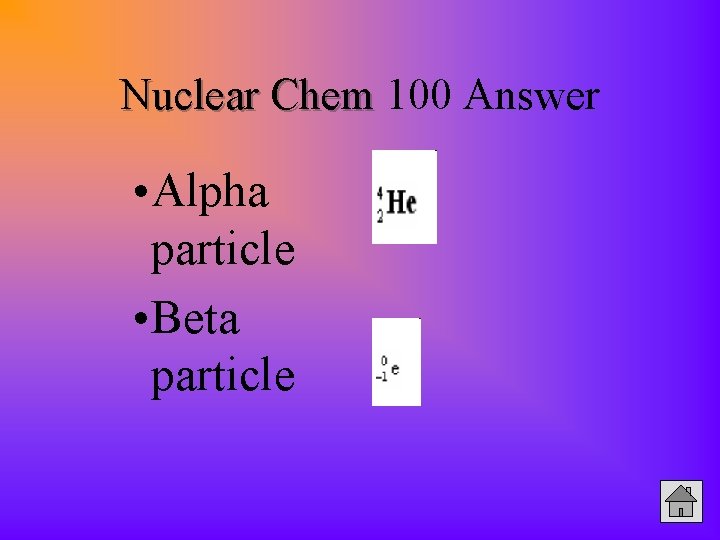
Nuclear Chem 100 Answer • Alpha particle • Beta particle

200 Nuclear Chem • What type of radioactive decay results in energy only being emitted from the nucleus? Answer

Nuclear Chem 200 • Gamma radiation

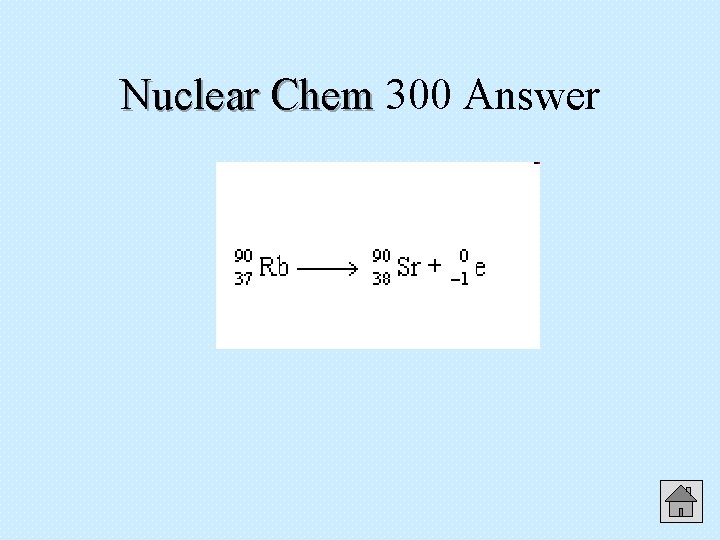
300 Nuclear Chem Write the nuclear chemical equation for the beta decay of rubidium-90. Answer

Nuclear Chem 300 Answer

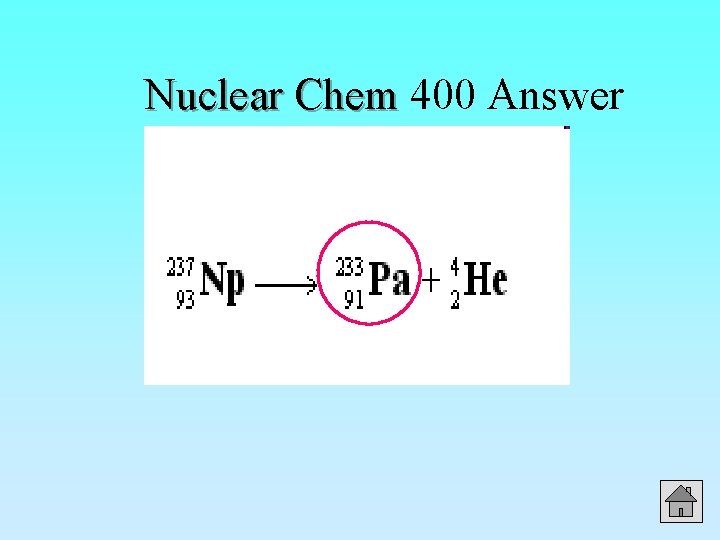
400 Nuclear Chem Write the nuclear symbol of the new element produced when Neptunium-237 (Np) undergoes alpha decay. Answer

Nuclear Chem 400 Answer

500 Nuclear Chem What scientist discovered the nucleus of the atom and later the proton? What was his/her country of origin? Answer

Nuclear Chem 500 Ernest Rutherford of New Zealand

100 Particles Name the particle that has about the same mass as a proton but no electric charge. Answer

Particles 100 Answer Neutron

200 Particles Write the complete nuclear symbol for the particle that has 48 protons 64 neutrons 46 electrons Answer

Particles 200 Answer

300 Particles Give the number of protons, neutrons and electrons in potassium-40. Answer

Particles 300 Answer 19 protons 21 neutrons 19 electrons

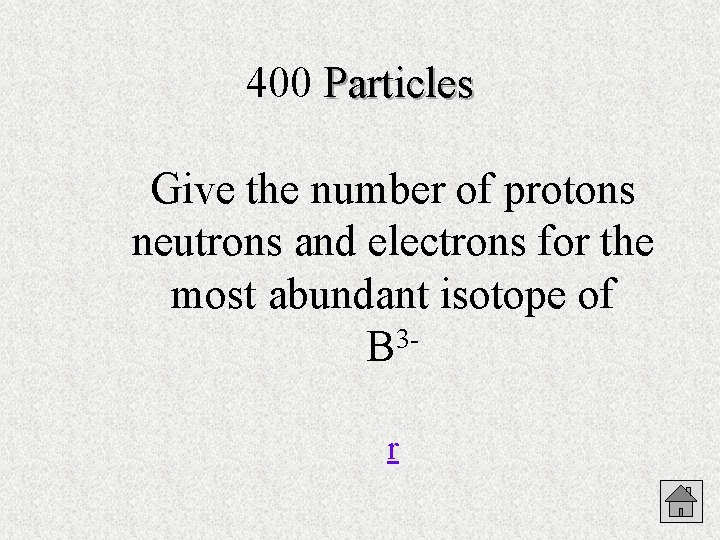
400 Particles Give the number of protons neutrons and electrons for the most abundant isotope of 3 B r

Particles 400 5 protons 6 neutrons 8 electrons

500 Particles Name the force which holds together the particles in the nucleus. Explain why it is strange to us. Answer

Particles 500 Nuclear Force It is strange because like charges usually repel, yet many positive charges are held together in small space.

100 Potpourri How many atoms -3 are in 7. 8 x 10 moles of zinc? Answer

Potpourri 100 Using Avogadro’s number as the conversion factor: ? Atoms = 7. 8 x 10 -3 mol X 6. 02 x 1023 atoms mol = 4. 7 x 1021 atoms

200 Potpourri How many moles are there in 50. 5 g of neon? Answer

Answers for Potpourri 200 Using molar mass of Ne = 20. 18 g/mol ? Mol = 50. 5 g x 1 mol = 2. 50 mol 20. 18 g

300 Potpourri Name the two main regions of the atom. Which region occupies most of the volume of the atom. Which region has most of the atom’s mass? Answer

Potpourri 300 • The two regions are the nucleus and the electron cloud. • The electron cloud constitutes most of the volume of the atom and is mostly empty space. • The nucleus contains most of the atom’s mass.

400 Potpourri A reaction between 46 g of sodium and 71 g of chlorine will produce how much salt? Answer

Potpourri 400 Answer 117 g (Law of Conservation of Mass)

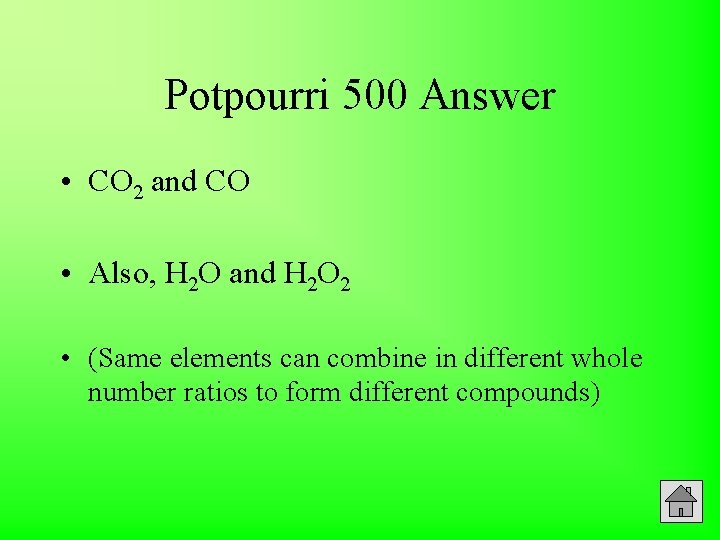
500 Potpourri Give chemical formulas of two compounds which support the Law of Multiple Proportions. Answer

Potpourri 500 Answer • CO 2 and CO • Also, H 2 O and H 2 O 2 • (Same elements can combine in different whole number ratios to form different compounds)

Final Jeopardy What is the mass of exactly one iron atom? Answer

Final Jeopardy Answer First convert one atom to moles. ? Mole = 1 atom X 1 mole = 1. 6611 x 10 -24 mol 6. 02 x 1023 atoms Then convert moles to grams using molar mass. ? g = 1. 6611 x 10 -24 mol X 55. 85 g = 9. 28 x 10 -23 g mole