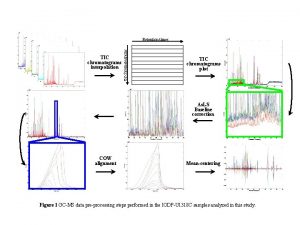
Figure S 1 DAD chromatograms of carotenoids mixture


- Slides: 13

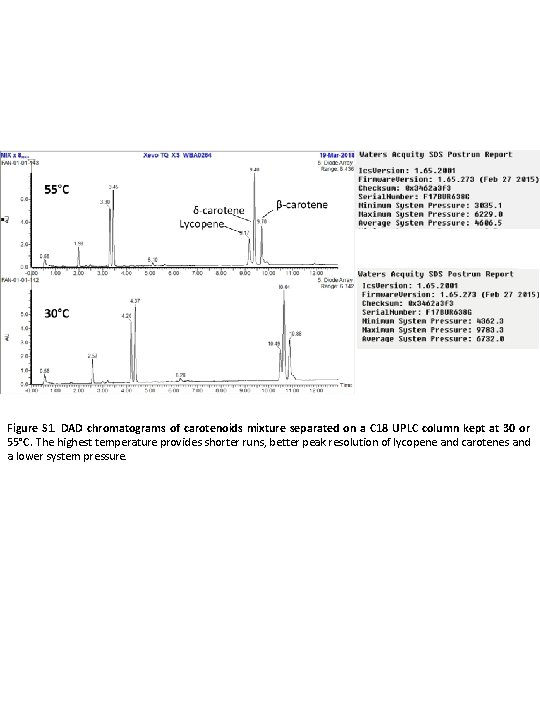
Figure S 1. DAD chromatograms of carotenoids mixture separated on a C 18 UPLC column kept at 30 or 55°C. The highest temperature provides shorter runs, better peak resolution of lycopene and carotenes and a lower system pressure.

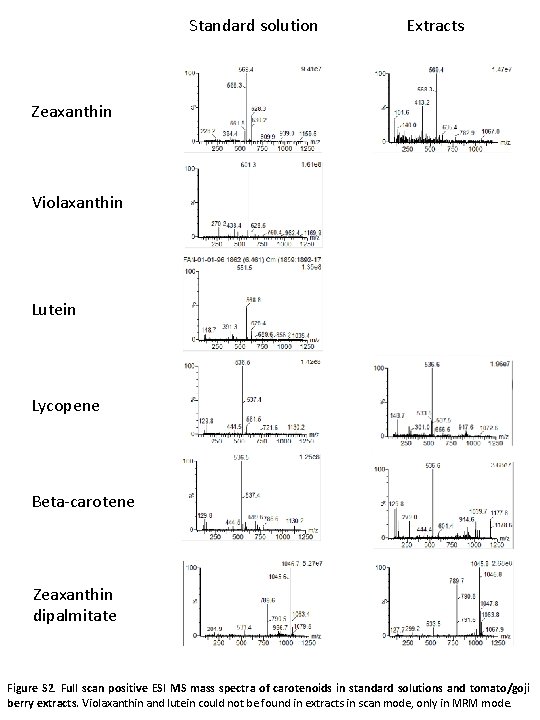
Standard solution Extracts Zeaxanthin Violaxanthin Lutein Lycopene Beta-carotene Zeaxanthin dipalmitate Figure S 2. Full scan positive ESI MS mass spectra of carotenoids in standard solutions and tomato/goji berry extracts. Violaxanthin and lutein could not be found in extracts in scan mode, only in MRM mode.

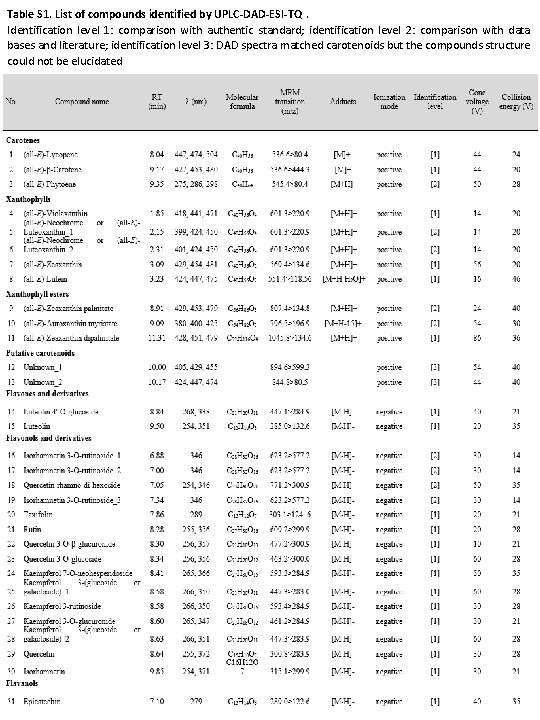
Table S 1. List of compounds identified by UPLC-DAD-ESI-TQ. Identification level 1: comparison with authentic standard; identification level 2: comparison with data bases and literature; identification level 3: DAD spectra matched carotenoids but the compounds structure could not be elucidated

Table S 1. (continued)

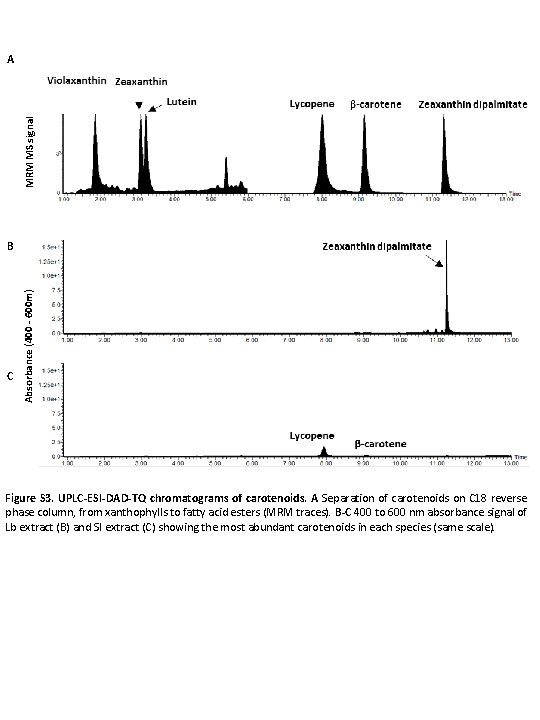
MRM MS signal A C Absorbance (400 - 600 m) B Figure S 3. UPLC-ESI-DAD-TQ chromatograms of carotenoids. A Separation of carotenoids on C 18 reverse phase column, from xanthophylls to fatty acid esters (MRM traces). B-C 400 to 600 nm absorbance signal of Lb extract (B) and Sl extract (C) showing the most abundant carotenoids in each species (same scale).

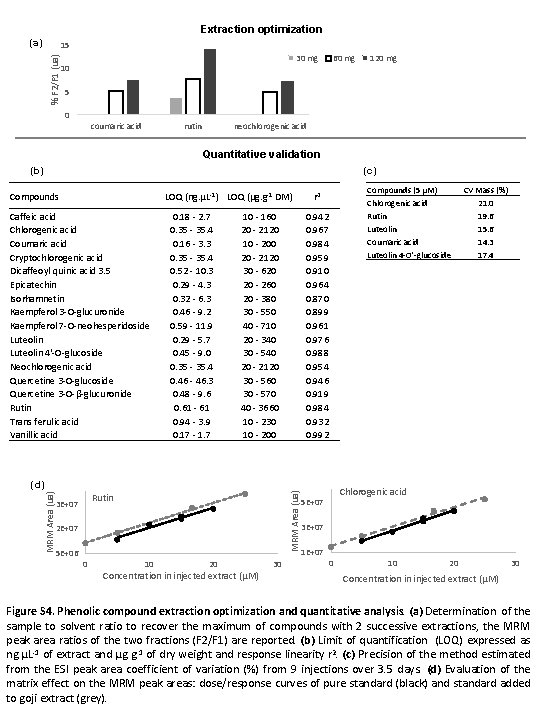
Extraction optimization (a) % F 2/F 1 (ua) 15 30 mg 10 60 mg 120 mg 5 0 coumaric acid rutin neochlorogenic acid Quantitative validation (b) (c) Compounds LOQ (ng. µL-1) LOQ (µg. g-1 DM) Caffeic acid Chlorogenic acid Coumaric acid Cryptochlorogenic acid Dicaffeoyl quinic acid 3. 5 Epicatechin Isorhamnetin Kaempferol 3 -O-glucuronide Kaempferol 7 -O-neohesperidoside Luteolin 4'-O-glucoside Neochlorogenic acid Quercetine 3 -O-glucoside Quercetine 3 -O-β-glucuronide Rutin Trans ferulic acid Vanillic acid 0. 18 - 2. 7 0. 35 - 35. 4 0. 16 - 3. 3 0. 35 - 35. 4 0. 52 - 10. 3 0. 29 - 4. 3 0. 32 - 6. 3 0. 46 - 9. 2 0. 59 - 11. 9 0. 29 - 5. 7 0. 45 - 9. 0 0. 35 - 35. 4 0. 46 - 46. 3 0. 48 - 9. 6 0. 61 - 61 0. 94 - 3. 9 0. 17 - 1. 7 10 - 160 20 - 2120 10 - 200 20 - 2120 30 - 620 20 - 260 20 - 380 30 - 550 40 - 710 20 - 340 30 - 540 20 - 2120 30 - 560 30 - 570 40 - 3660 10 - 230 10 - 200 Rutin 3 E+07 2 E+07 5 E+06 0 0. 942 0. 967 0. 984 0. 959 0. 910 0. 964 0. 870 0. 899 0. 961 0. 976 0. 988 0. 954 0. 946 0. 919 0. 984 0. 932 0. 992 MRM Area (ua) (d) 10 20 Concentration in injected extract (µM) 30 Compounds (5 µM) Chlorogenic acid Rutin Luteolin Coumaric acid Luteolin 4 -O'-glucoside r 2 CV Mass (%) 21. 0 19. 6 15. 6 14. 3 17. 4 Chlorogenic acid 5 E+07 3 E+07 1 E+07 0 10 20 30 Concentration in injected extract (µM) Figure S 4. Phenolic compound extraction optimization and quantitative analysis. (a) Determination of the sample to solvent ratio to recover the maximum of compounds with 2 successive extractions, the MRM peak area ratios of the two fractions (F 2/F 1) are reported. (b) Limit of quantification (LOQ) expressed as ng. µL-1 of extract and µg. g-1 of dry weight and response linearity r 2. (c) Precision of the method estimated from the ESI peak area coefficient of variation (%) from 9 injections over 3. 5 days. (d) Evaluation of the matrix effect on the MRM peak areas: dose/response curves of pure standard (black) and standard added to goji extract (grey).

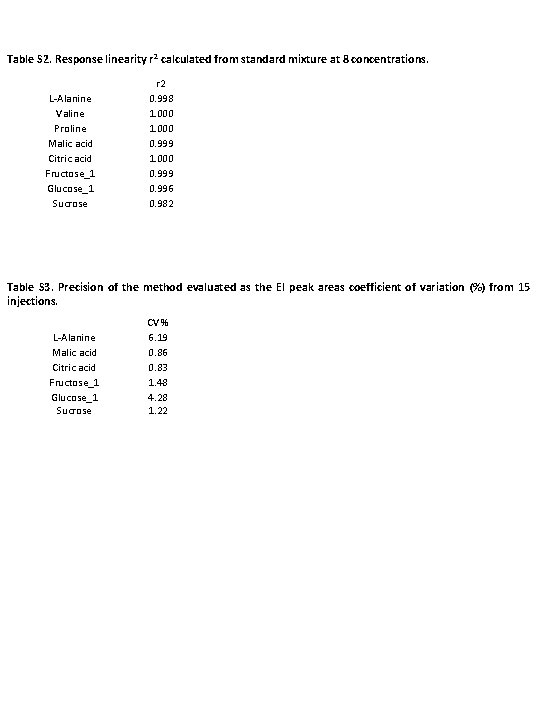
Table S 2. Response linearity r 2 calculated from standard mixture at 8 concentrations. L-Alanine Valine Proline Malic acid Citric acid Fructose_1 Glucose_1 Sucrose r 2 0. 998 1. 000 0. 999 0. 996 0. 982 Table S 3. Precision of the method evaluated as the EI peak areas coefficient of variation (%) from 15 injections. L-Alanine Malic acid Citric acid Fructose_1 Glucose_1 Sucrose CV % 6. 19 0. 86 0. 83 1. 48 4. 28 1. 22

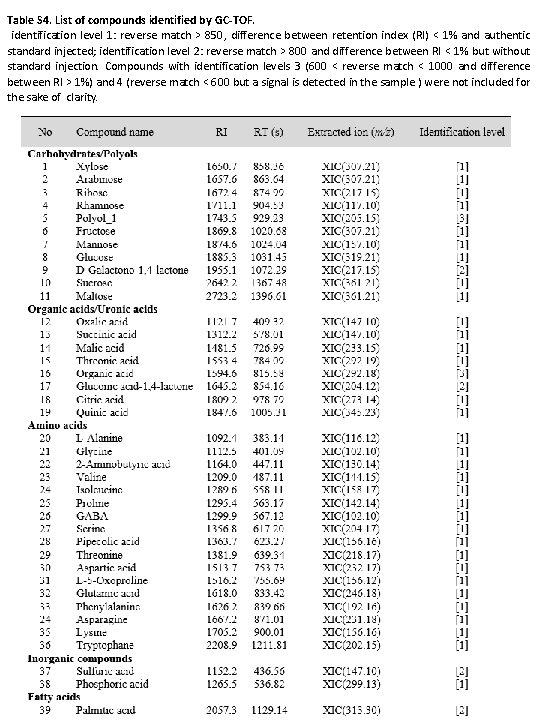
Table S 4. List of compounds identified by GC-TOF. identification level 1: reverse match > 850, difference between retention index (RI) < 1% and authentic standard injected; identification level 2: reverse match > 800 and difference between RI < 1% but without standard injection. Compounds with identification levels 3 (600 < reverse match < 1000 and difference between RI > 1%) and 4 (reverse match < 600 but a signal is detected in the sample ) were not included for the sake of clarity.

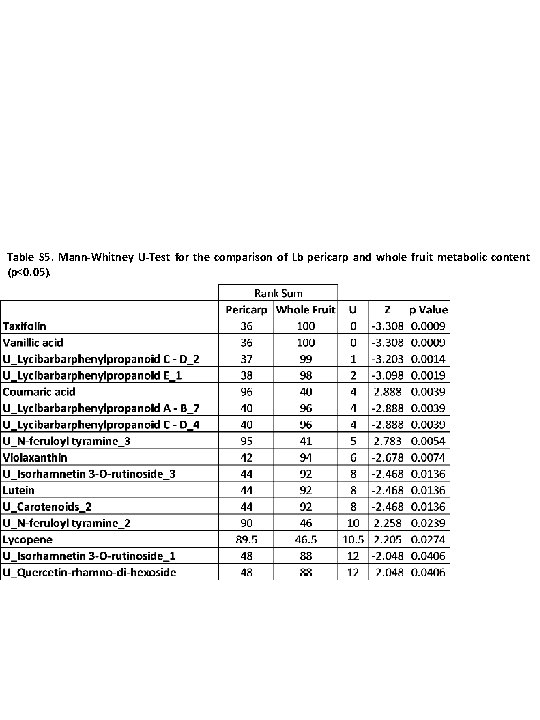
Table S 5. Mann-Whitney U-Test for the comparison of Lb pericarp and whole fruit metabolic content (p<0. 05).

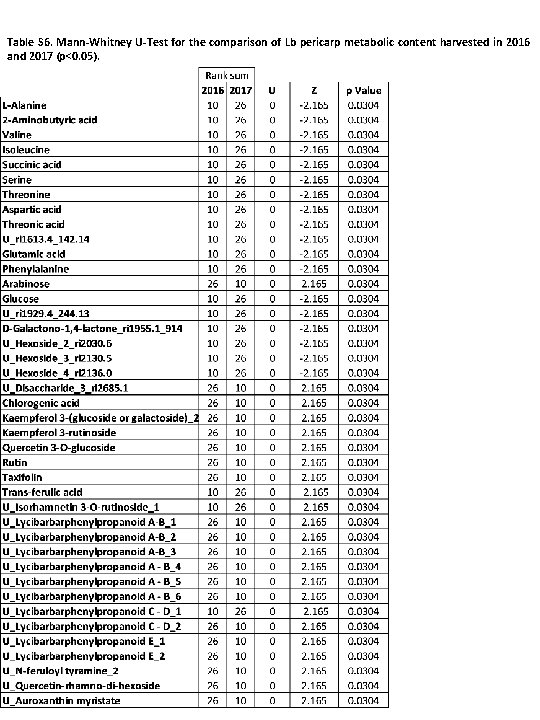
Table S 6. Mann-Whitney U-Test for the comparison of Lb pericarp metabolic content harvested in 2016 and 2017 (p<0. 05).

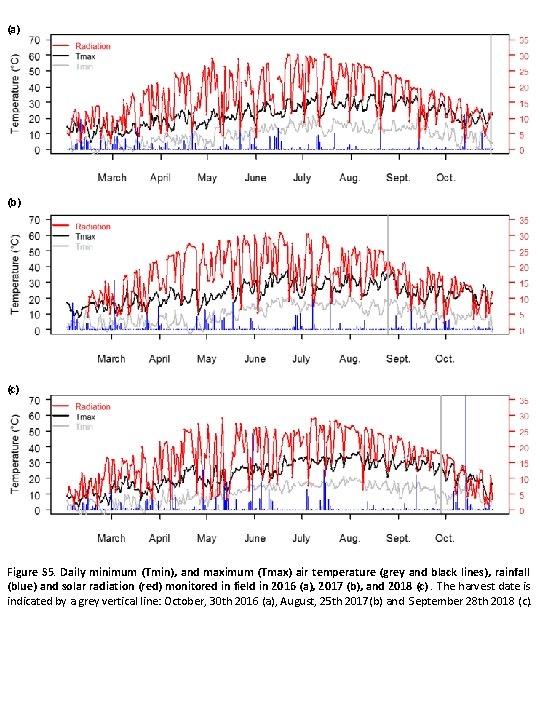
(a) (b) (c) Figure S 5. Daily minimum (Tmin), and maximum (Tmax) air temperature (grey and black lines), rainfall (blue) and solar radiation (red) monitored in field in 2016 (a), 2017 (b), and 2018 (c). The harvest date is indicated by a grey vertical line: October, 30 th 2016 (a), August, 25 th 2017(b) and September 28 th 2018 (c).

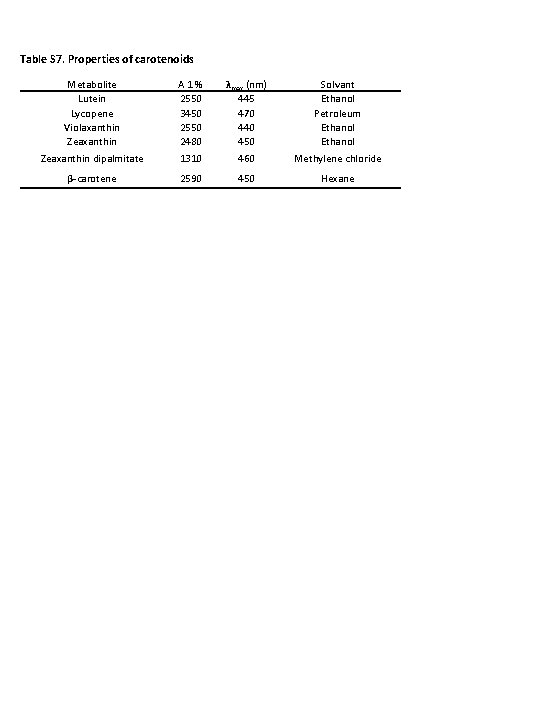
Table S 7. Properties of carotenoids Metabolite Lutein Lycopene Violaxanthin Zeaxanthin A 1 % 2550 3450 2550 2480 λmax (nm) 445 470 440 450 Solvant Ethanol Petroleum Ethanol Zeaxanthin dipalmitate 1310 460 Methylene chloride β-carotene 2590 450 Hexane

Table S 8. List of the tests performed for method optimization, covering three metabolite classes. Parameters Carotenoids Extraction yield • Ratio fruit powder / volume of • Ratio fruit powder / volume extraction solvent (85, 110 of extraction solvent (17, of extraction solvent (3, 5, mg. m. L-1) 33, 50 et 67 mg. m. L-1) 13 mg. m. L-1) Metabolite stability Phenolic compounds • Number successive extractions • Number of successive extractions • Use Ca. CO 3 in extraction solvent • Formic acid in the extraction solvent Primary metabolites • Antioxidant concentration in the extraction solvent (BHT 0. 1 g. L 1 ; BHT 0. 2 g. L-1, BHT+BHA 0. 1 g. L-1) • % of MTBE in the injection solvent (16. 7 %, 33. 3 %) and composition of injection solvent (MTBE with Et. OH or ACN or Me. OH) • Sampler temperature (10°C, 20°C) Chromatography • Column(BEH C 18, HSS T 3, CSH • Column (CSH C 18, F 5) C 18, kinetex EVO C 18) • Mobile phases (water/ACN, • Mobile phases (water/ACN, water/Me. OH) and elution gradient • Column temperature (30°C to 55°C) MRM parameters • Cone voltage (V) • Collision energy (e. V) Objective Carotenoids Method validation • Removal of cross-contamination (THF, multiple rinsing steps after the run) • Precision • Matrix effect • Quantification (linearity, LOD, LOQ) Phenolic compounds • Removal of cross-contamination (multiple rinsing steps after the run) • Precision • Matrix effect • Quantification (linearity, LOD, LOQ) Primary metabolites -Precision -Matrix effect
 Rich dad poor dad expense sheet
Rich dad poor dad expense sheet Homogeneous mixture and heterogeneous mixture class 9
Homogeneous mixture and heterogeneous mixture class 9 Identify the type of congruence transformation
Identify the type of congruence transformation 8 figure grid reference example
8 figure grid reference example Figure abcde is similar to figure vwxyz
Figure abcde is similar to figure vwxyz Start area
Start area Dad detektor
Dad detektor Rachel carson dad
Rachel carson dad Picture of mom and dad
Picture of mom and dad Breadwinner main character
Breadwinner main character Beth putnam the crucible
Beth putnam the crucible Dreaming black boy poem questions and answers
Dreaming black boy poem questions and answers Your dad did what poem analysis
Your dad did what poem analysis Riparte la dad
Riparte la dad