Figure 6 1 Figure 6 UN 01 Enzyme








- Slides: 18

Figure 6. 1

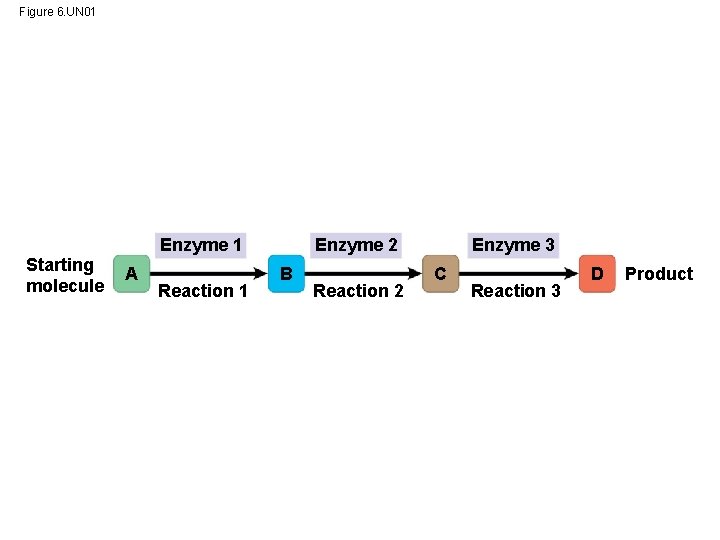
Figure 6. UN 01 Enzyme 1 Starting molecule A Reaction 1 Enzyme 2 B Reaction 2 Enzyme 3 C Reaction 3 D Product

Figure 6. 2 A diver has more potential energy on the platform. Climbing up converts the kinetic energy of muscle movement to potential energy. Diving converts potential energy to kinetic energy. A diver has less potential energy in the water.

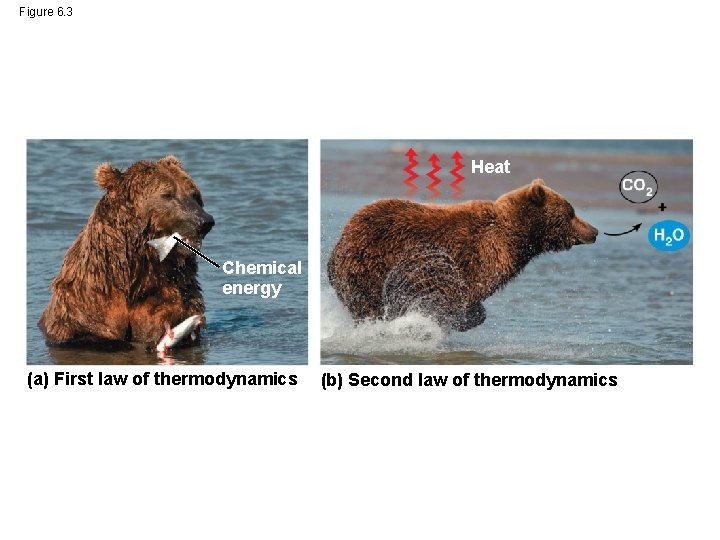
Figure 6. 3 Heat Chemical energy (a) First law of thermodynamics (b) Second law of thermodynamics

Figure 6. 4

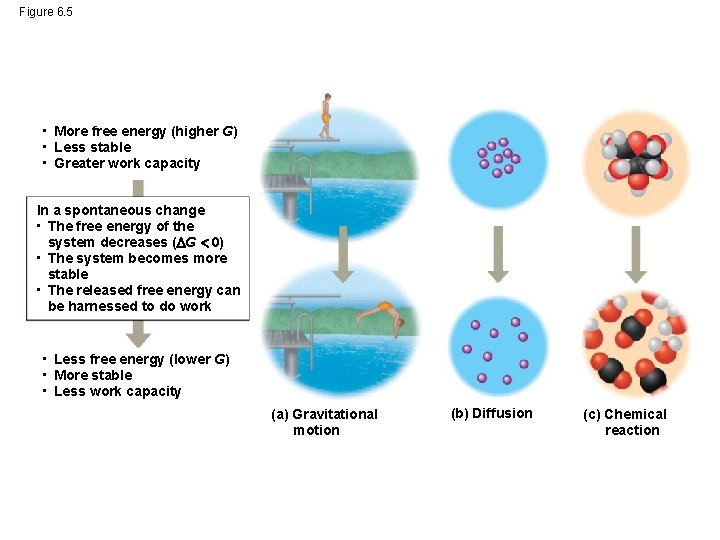
Figure 6. 5 • More free energy (higher G) • Less stable • Greater work capacity In a spontaneous change • The free energy of the system decreases ( G 0) • The system becomes more stable • The released free energy can be harnessed to do work • Less free energy (lower G) • More stable • Less work capacity (a) Gravitational motion (b) Diffusion (c) Chemical reaction

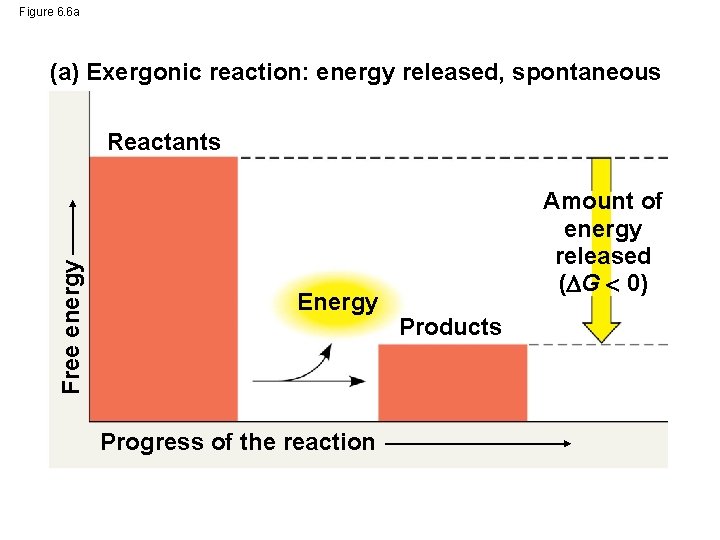
Figure 6. 6 a (a) Exergonic reaction: energy released, spontaneous Free energy Reactants Energy Progress of the reaction Amount of energy released ( G 0) Products

Figure 6. 6 b (b) Endergonic reaction: energy required, nonspontaneous Free energy Products Reactants Energy Progress of the reaction Amount of energy required ( G 0)

Figure 6. 7 a G 0 (a) An isolated hydroelectric system G 0

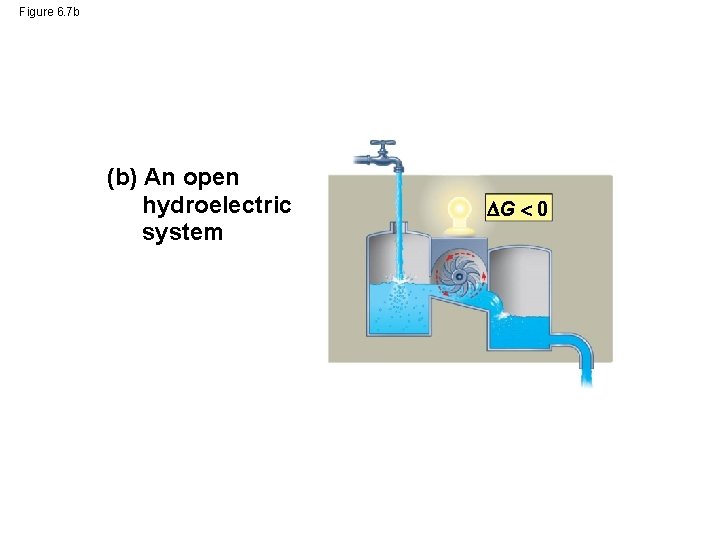
Figure 6. 7 b (b) An open hydroelectric system G 0

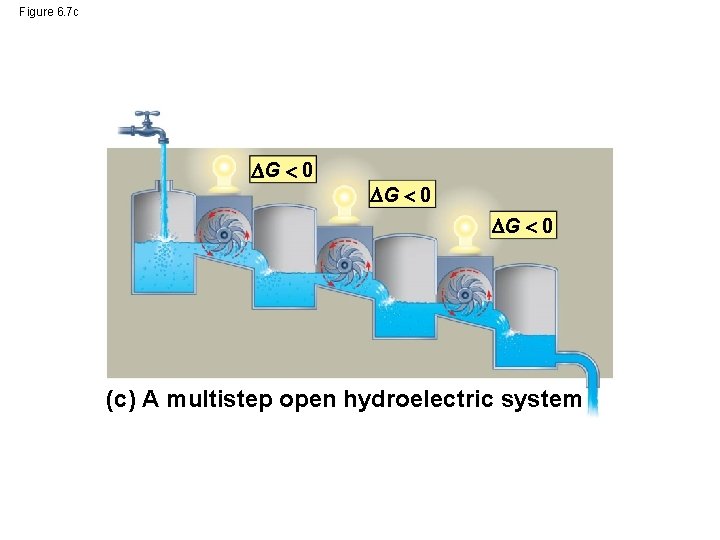
Figure 6. 7 c G 0 (c) A multistep open hydroelectric system

Figure 6. 8 a Adenine Phosphate groups (a) The structure of ATP Ribose

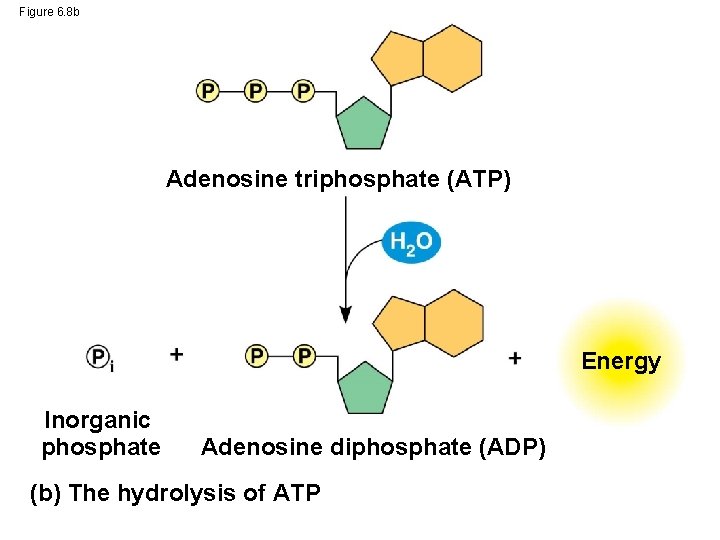
Figure 6. 8 b Adenosine triphosphate (ATP) Energy Inorganic phosphate Adenosine diphosphate (ADP) (b) The hydrolysis of ATP

Figure 6. 7 c G 0 (c) A multistep open hydroelectric system

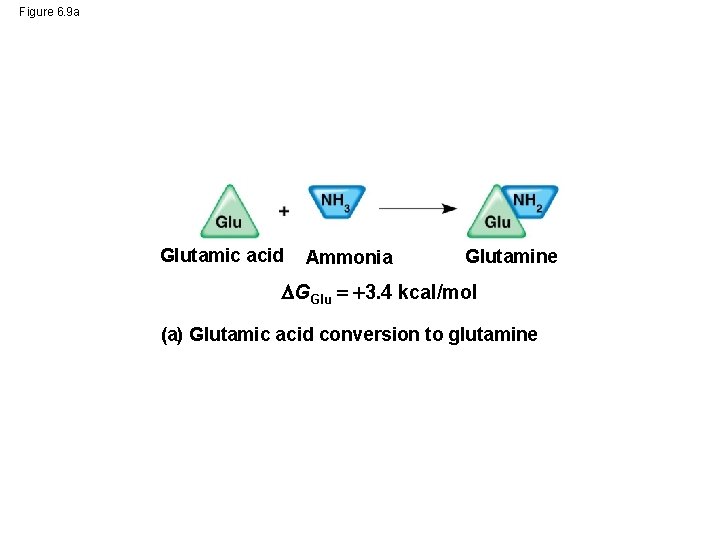
Figure 6. 9 a Glutamic acid Ammonia Glutamine GGlu 3. 4 kcal/mol (a) Glutamic acid conversion to glutamine

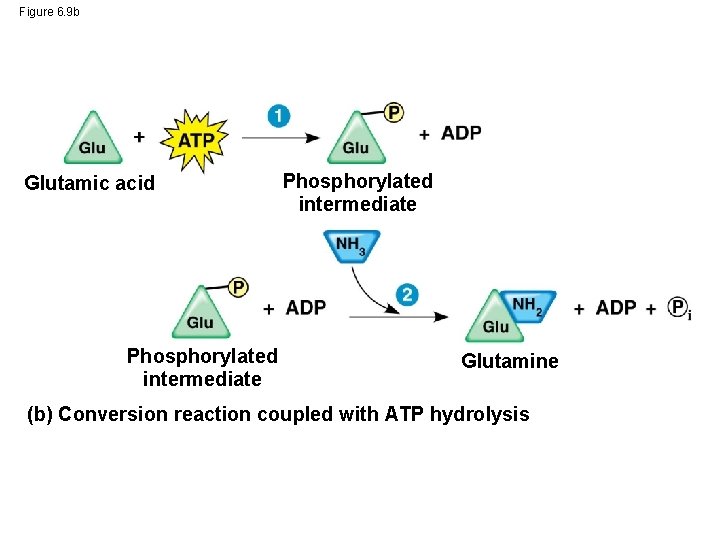
Figure 6. 9 b Glutamic acid Phosphorylated intermediate Glutamine (b) Conversion reaction coupled with ATP hydrolysis

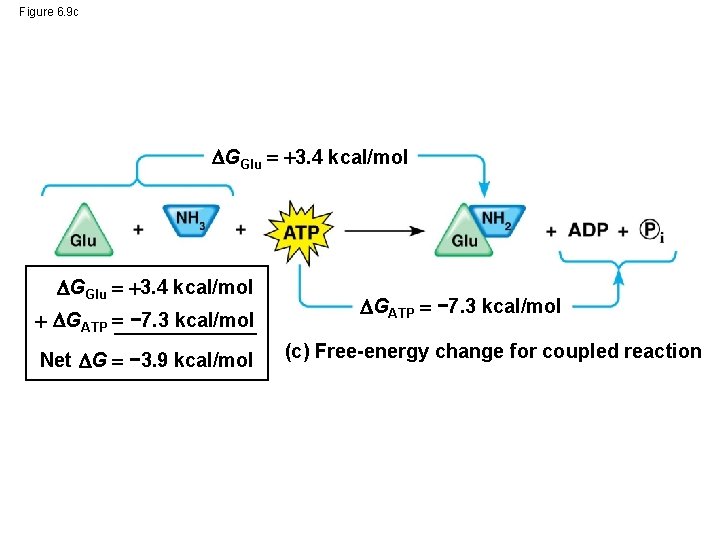
Figure 6. 9 c GGlu 3. 4 kcal/mol GATP − 7. 3 kcal/mol Net G − 3. 9 kcal/mol GATP − 7. 3 kcal/mol (c) Free-energy change for coupled reaction

Figure 6. 11 Energy from catabolism (exergonic, energyreleasing processes) Energy for cellular work (endergonic, energy-consuming processes)
 Factors affecting enzyme activity slideshare
Factors affecting enzyme activity slideshare Enzyme-linked immunosorbent assay (elisa)
Enzyme-linked immunosorbent assay (elisa) Enzyme present in mouth
Enzyme present in mouth Hydrophilic heads
Hydrophilic heads Tunesflux
Tunesflux What makes an enzyme specific
What makes an enzyme specific Modul eco enzyme
Modul eco enzyme Inhibitor vs inducer
Inhibitor vs inducer Molecular scissors
Molecular scissors Class of enzyme
Class of enzyme Keplas
Keplas What is enzyme induction
What is enzyme induction Brush border function
Brush border function The principle enzyme involved in dna replication is called
The principle enzyme involved in dna replication is called Lipase enzyme source
Lipase enzyme source The diagram represents one way an enzyme can be inhibited
The diagram represents one way an enzyme can be inhibited Enzymes affect the reaction in living cells by changing the
Enzymes affect the reaction in living cells by changing the Protein purification
Protein purification Allosteric enzyme
Allosteric enzyme