Fig 7 CO p 184 Fig 7 1





- Slides: 29

Fig. 7 -CO, p. 184

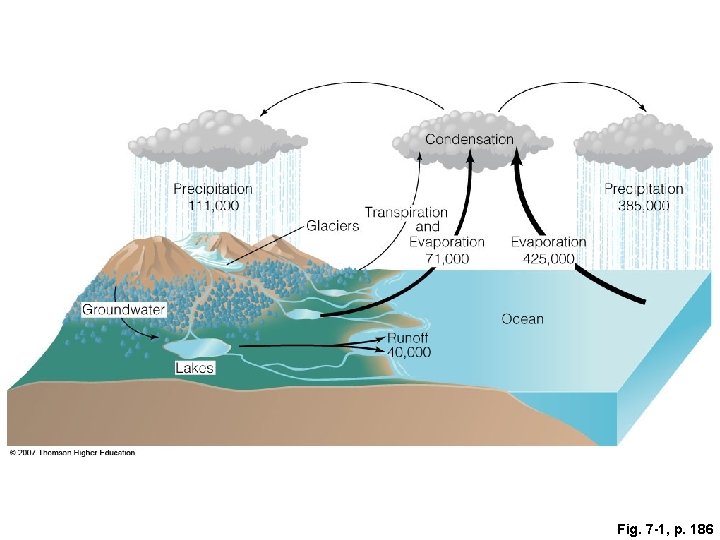
Fig. 7 -1, p. 186

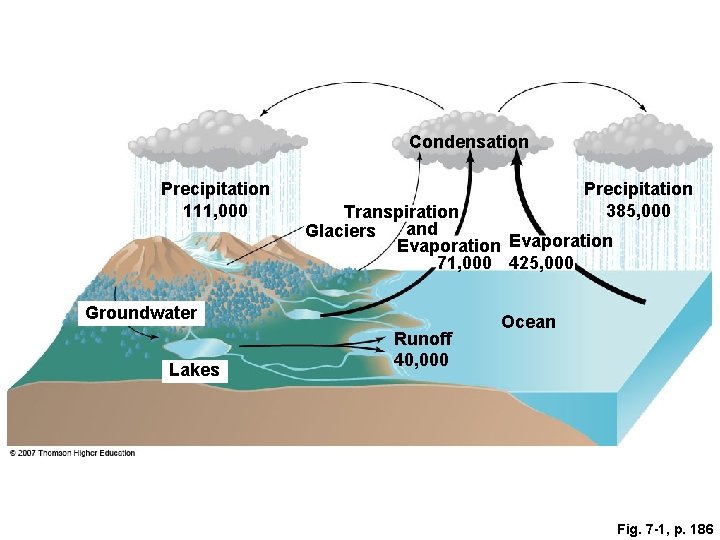
Condensation Precipitation 111, 000 Precipitation 385, 000 Transpiration and Glaciers Evaporation 71, 000 425, 000 Groundwater Lakes Runoff 40, 000 Ocean Fig. 7 -1, p. 186

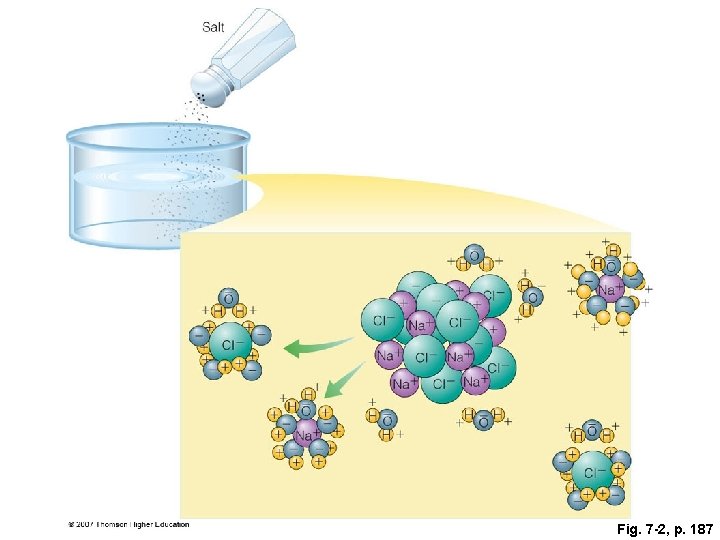
Fig. 7 -2, p. 187

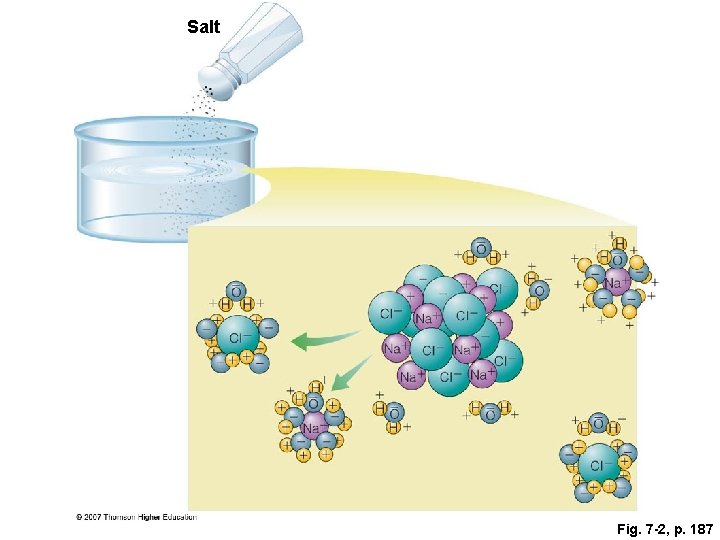
Salt Fig. 7 -2, p. 187

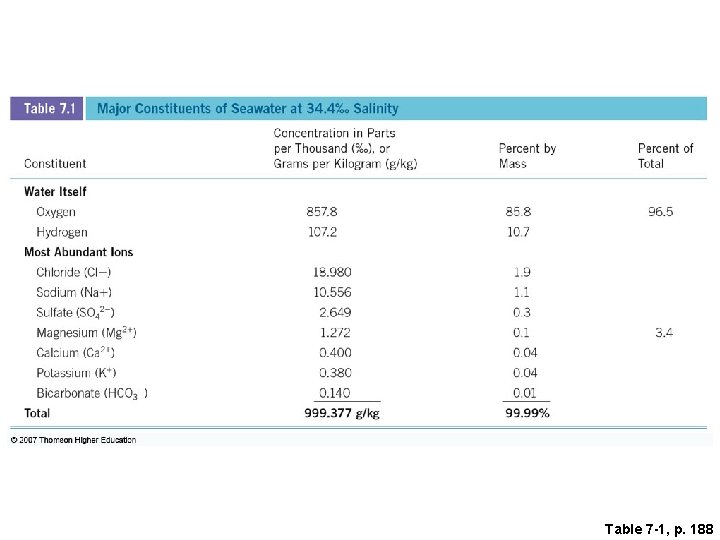
Table 7 -1, p. 188

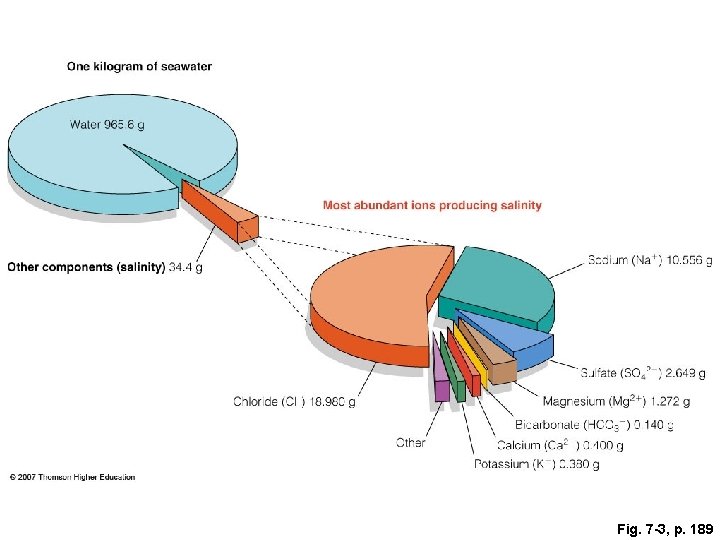
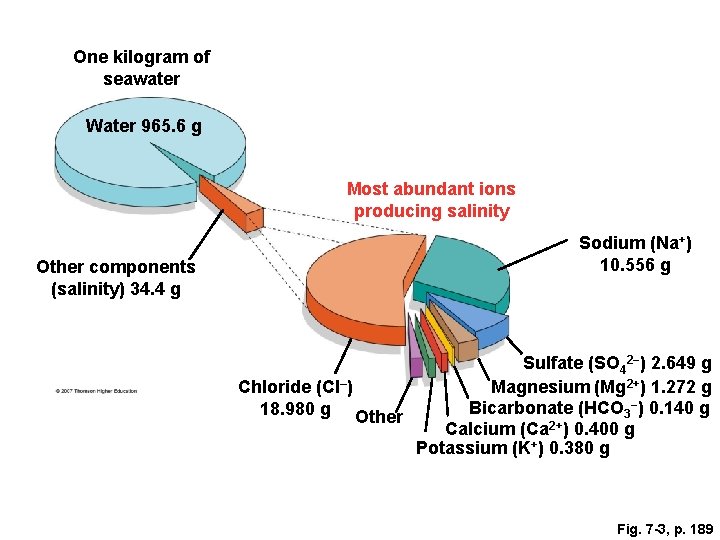
Fig. 7 -3, p. 189

One kilogram of seawater Water 965. 6 g Most abundant ions producing salinity Other components (salinity) 34. 4 g Sodium (Na+) 10. 556 g Sulfate (SO 42−) 2. 649 g Chloride (Cl–) Magnesium (Mg 2+) 1. 272 g Bicarbonate (HCO 3−) 0. 140 g 18. 980 g Other Calcium (Ca 2+) 0. 400 g Potassium (K+) 0. 380 g Fig. 7 -3, p. 189

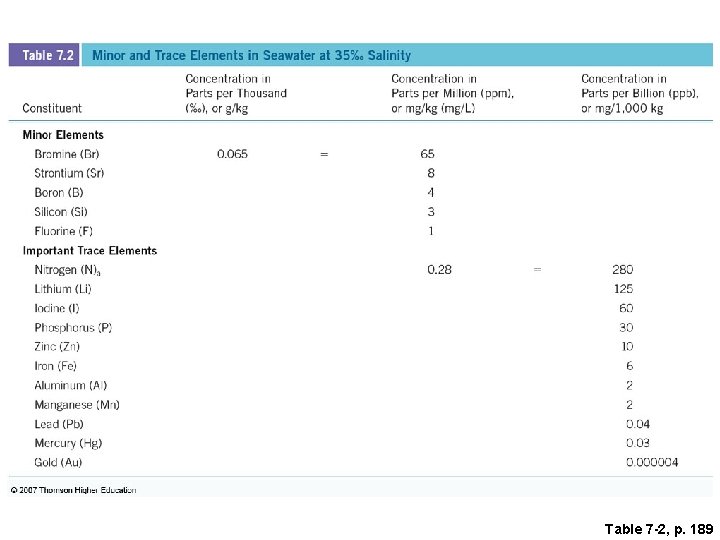
Table 7 -2, p. 189

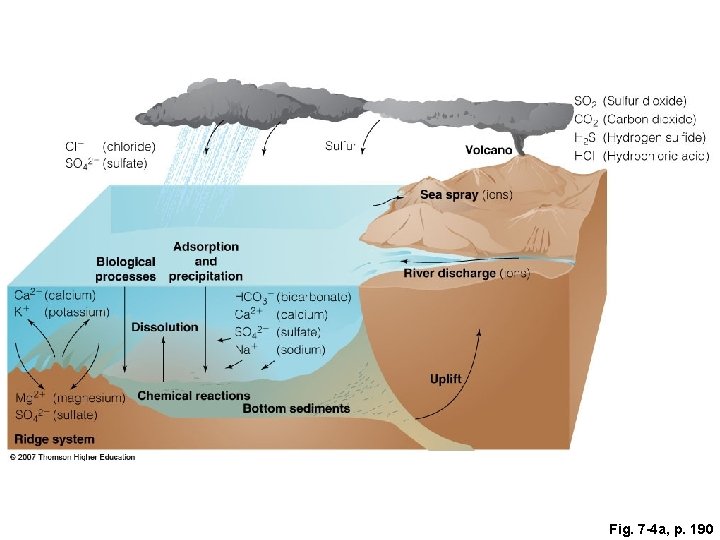
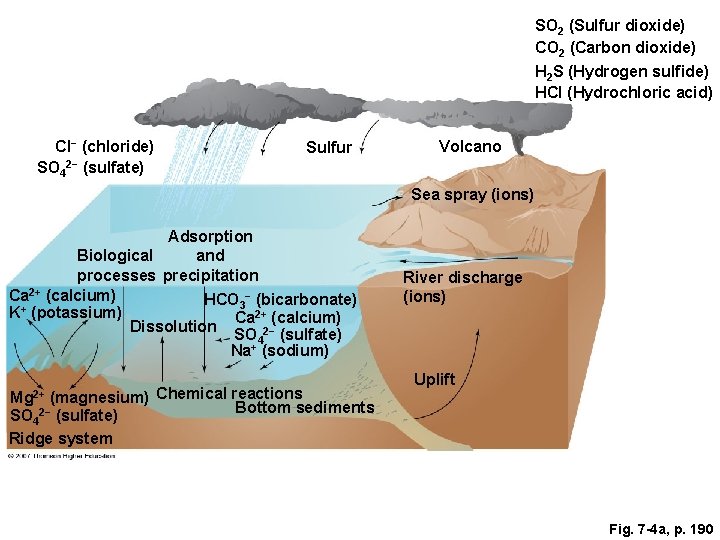
Fig. 7 -4 a, p. 190

SO 2 (Sulfur dioxide) CO 2 (Carbon dioxide) H 2 S (Hydrogen sulfide) HCl (Hydrochloric acid) Cl− (chloride) SO 42− (sulfate) Sulfur Volcano Sea spray (ions) Adsorption and Biological processes precipitation Ca 2+ (calcium) HCO 3− (bicarbonate) K+ (potassium) Ca 2+ (calcium) Dissolution SO 2− (sulfate) 4 Na+ (sodium) (magnesium) Chemical reactions Bottom sediments SO 42− (sulfate) Ridge system River discharge (ions) Uplift Mg 2+ Fig. 7 -4 a, p. 190

Fig. 7 -4 b, p. 190

Fig. 7 -5, p. 191

Fig. 7 -6, p. 192

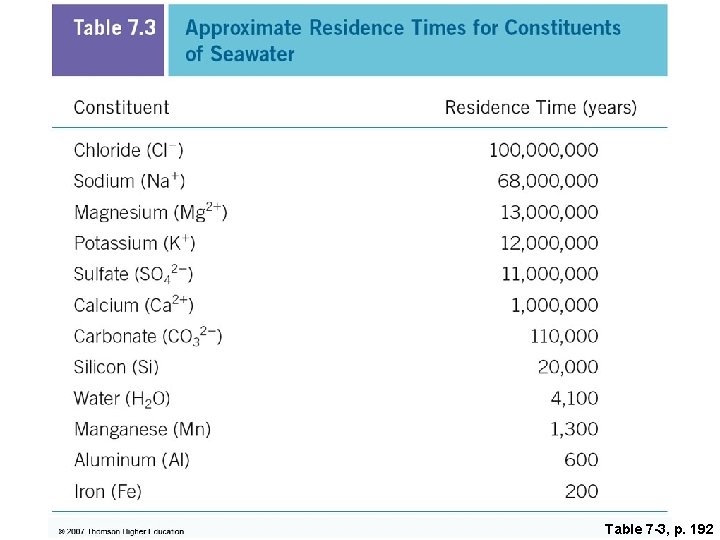
Table 7 -3, p. 192

Box 7 -1, p. 193

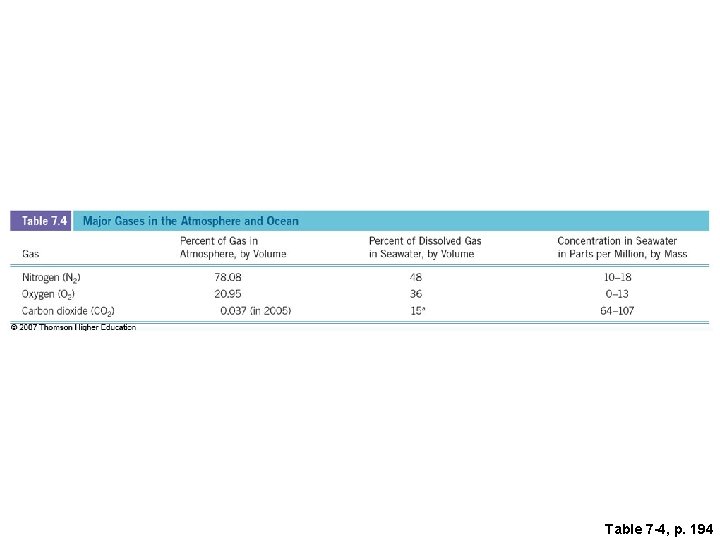
Table 7 -4, p. 194

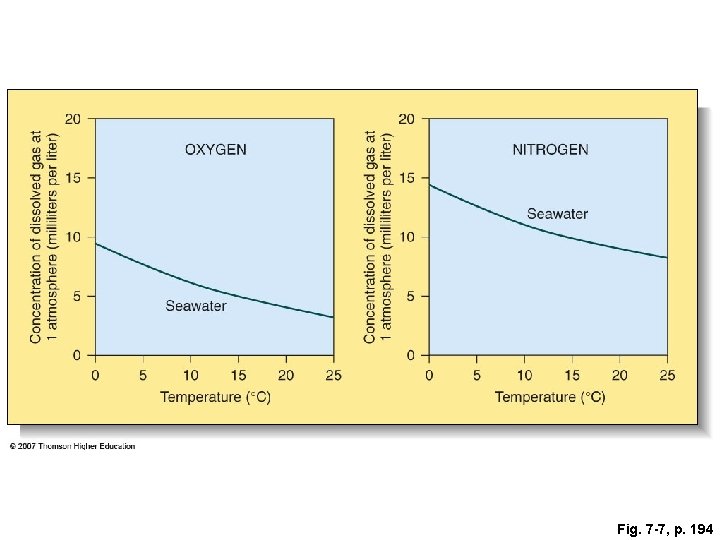
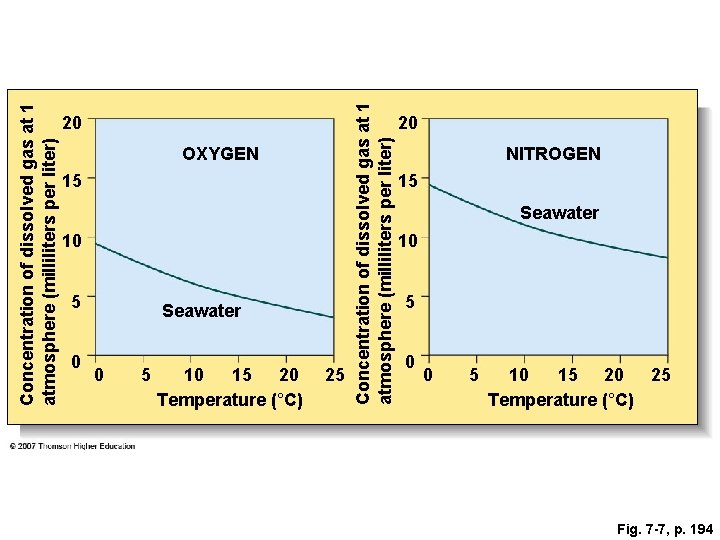
Fig. 7 -7, p. 194

Concentration of dissolved gas at 1 atmosphere (milliliters per liter) OXYGEN 15 10 5 0 Seawater 0 5 10 15 20 Temperature (°C) 25 Concentration of dissolved gas at 1 atmosphere (milliliters per liter) 20 20 NITROGEN 15 Seawater 10 5 0 0 5 10 15 20 25 Temperature (°C) Fig. 7 -7, p. 194

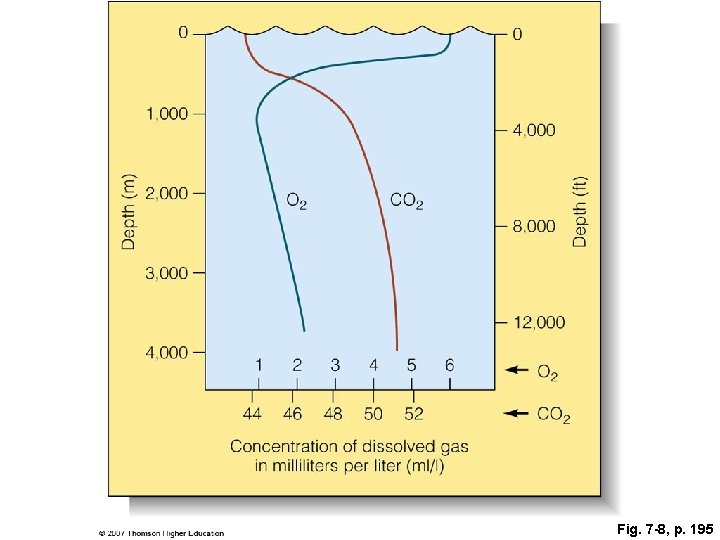
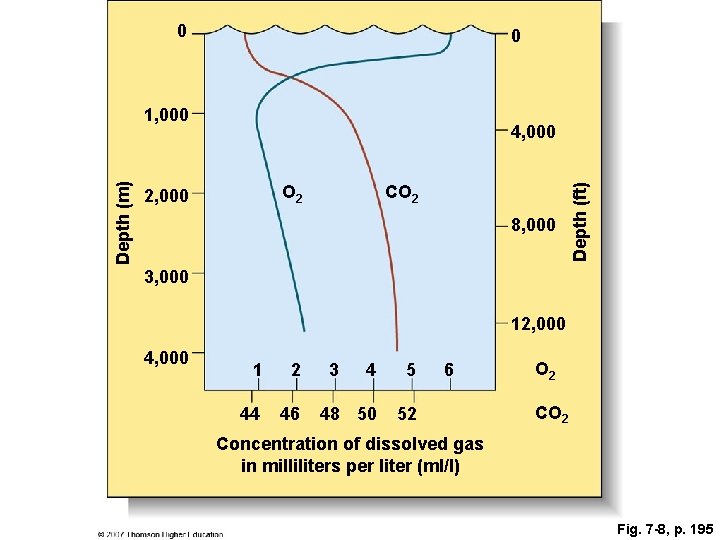
Fig. 7 -8, p. 195

0 0 4, 000 O 2 2, 000 CO 2 8, 000 Depth (ft) Depth (m) 1, 000 3, 000 12, 000 4, 000 1 2 44 46 3 4 5 48 50 52 6 O 2 Concentration of dissolved gas in milliliters per liter (ml/l) Fig. 7 -8, p. 195

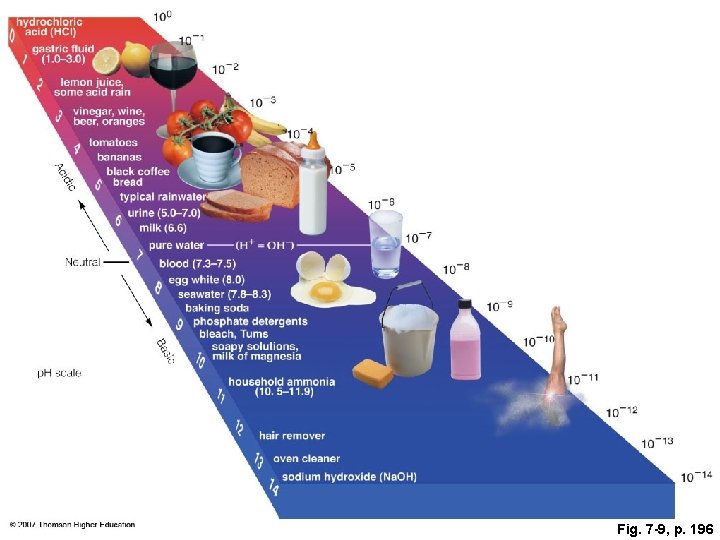
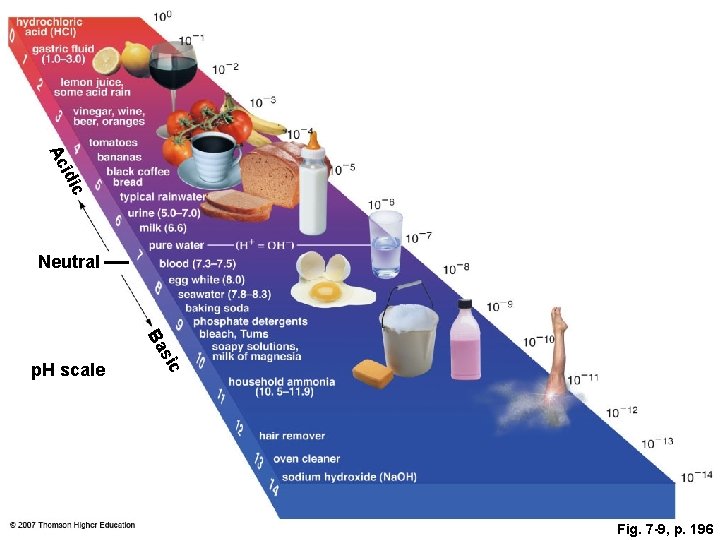
Fig. 7 -9, p. 196

Ac idi c Neutral ic s Ba p. H scale Fig. 7 -9, p. 196

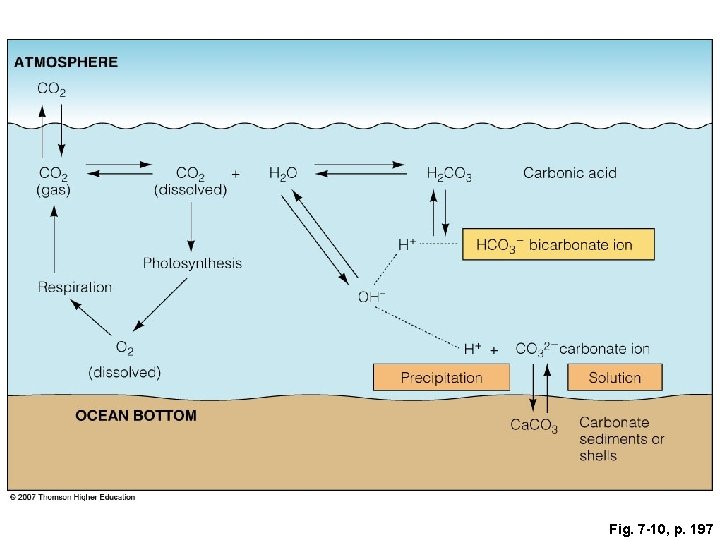
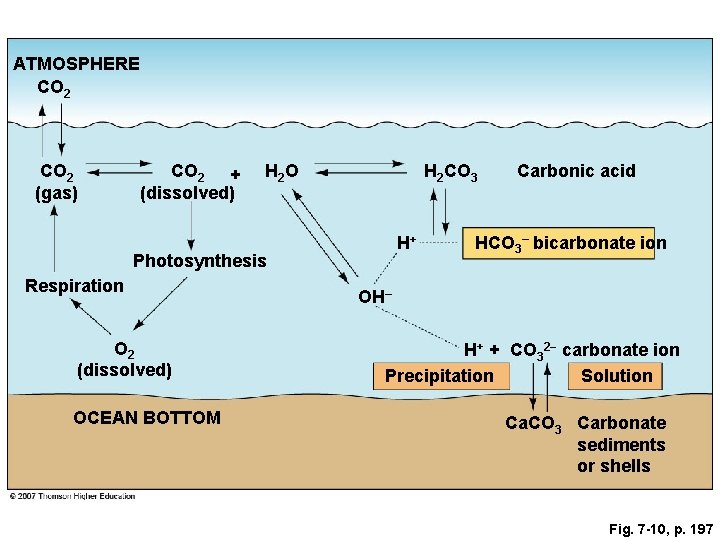
Fig. 7 -10, p. 197

ATMOSPHERE CO 2 (gas) CO 2 + (dissolved) H 2 O H 2 CO 3 H+ Photosynthesis Respiration O 2 (dissolved) OCEAN BOTTOM Carbonic acid HCO 3– bicarbonate ion OH– H+ + CO 32− carbonate ion Precipitation Solution Ca. CO 3 Carbonate sediments or shells Fig. 7 -10, p. 197

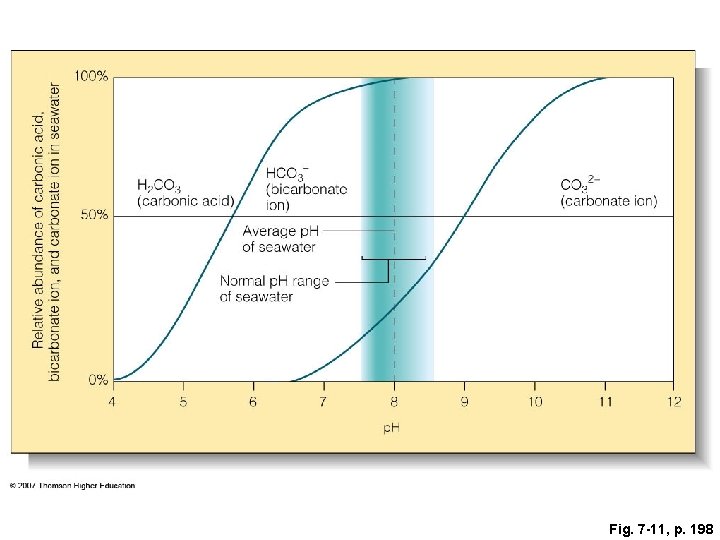
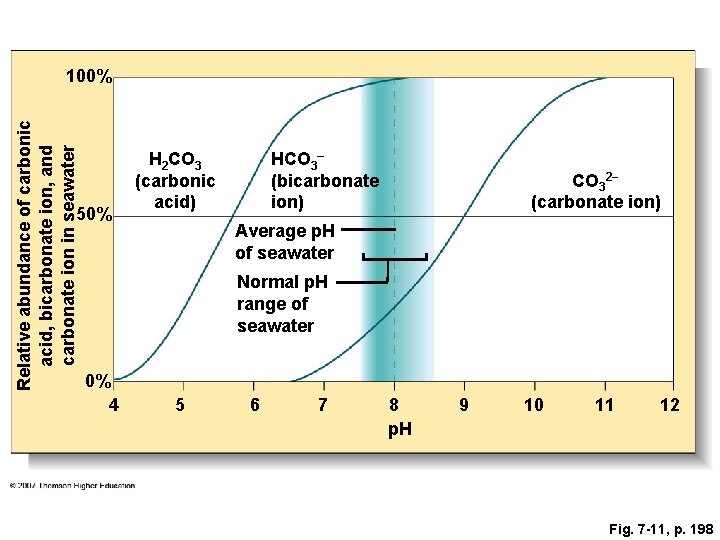
Fig. 7 -11, p. 198

Relative abundance of carbonic acid, bicarbonate ion, and carbonate ion in seawater 100% 50% H 2 CO 3 (carbonic acid) HCO 3– (bicarbonate ion) CO 32− (carbonate ion) Average p. H of seawater Normal p. H range of seawater 0% 4 5 6 7 8 p. H 9 10 11 12 Fig. 7 -11, p. 198

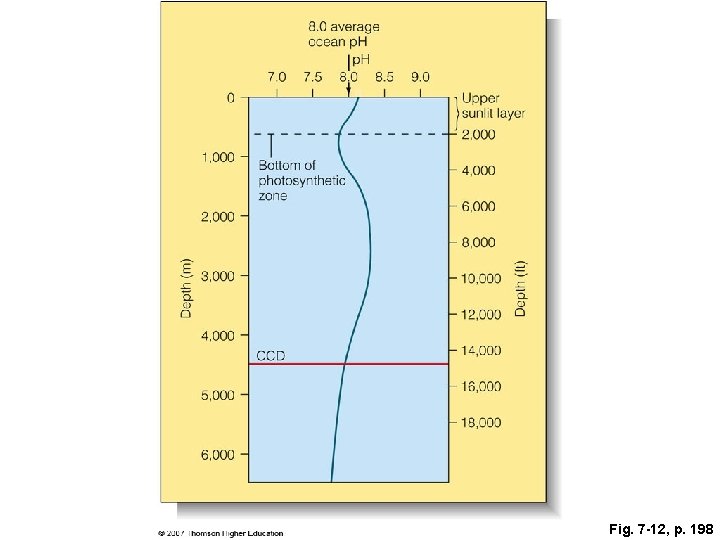
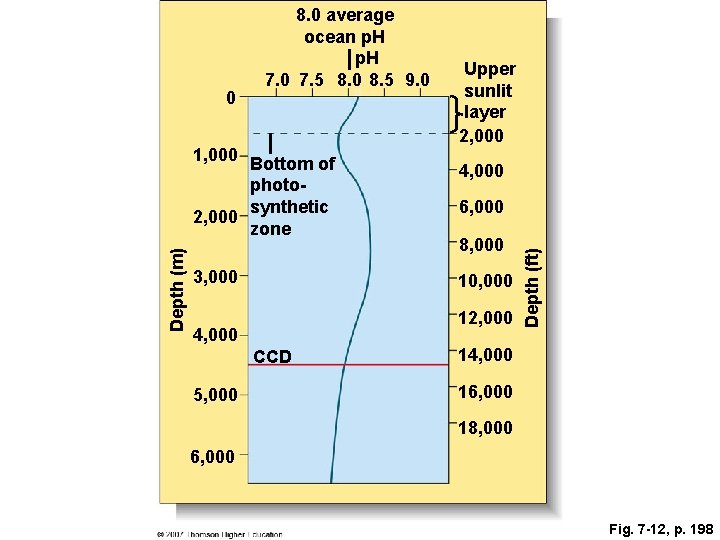
Fig. 7 -12, p. 198

Depth (m) 1, 000 Bottom of photosynthetic 2, 000 zone 3, 000 4, 000 6, 000 8, 000 10, 000 12, 000 4, 000 CCD 5, 000 Upper sunlit layer 2, 000 Depth (ft) 0 8. 0 average ocean p. H 7. 0 7. 5 8. 0 8. 5 9. 0 14, 000 16, 000 18, 000 6, 000 Fig. 7 -12, p. 198