FFP 3 Qualitative Fit Testing Course Content Medical





















- Slides: 29

FFP 3 Qualitative Fit Testing

Course Content • Medical Exemptions • The purpose of fit testing • When to use a FFP 3 mask • Other PPE required – Donning & Doffing • The qualitative fit testing process

Paris Entries Relating to Covid-19 Before we begin………. . It is imperative that covid-19 interventions are documented on Paris as this data is extrapolated and sent to NHS England. When writing a progress note relating to C-19, • select ‘add intervention’ or ‘multiple interventions’ if recording more than one in the same progress note. • Type the prefix ‘Cov’ and a list of Covid-19 interventions will appear in the drop down box. These are ‘Record swab’, ‘Record test results’, ‘Record recovery/transfer’ (see flow chart on intranet). • Select the appropriate intervention and save.

Pre-testing Factors - Medical Exemptions For more information https: //www. gov. uk/government/publications/guidance-on-shielding-and-protecting-extremelyvulnerable-persons-from-covid-19/guidance-on-shielding-and-protecting-extremely-vulnerable-personsfrom-covid-19 • • Asthma – daily use of prophylaxis inhalers Diabetics on insulin Heart disease Pregnant workers Immunocompromised workers Renal failure Long term health conditions

Medical Exemptions continued • Solid organ transplant recipients. • People with severe respiratory conditions including all cystic fibrosis, severe asthma and severe COPD. • People with rare diseases and inborn errors of metabolism that significantly increase the risk of infections (such as SCID, homozygous sickle cell). • People on immunosuppression therapies sufficient to significantly increase risk of infection. • Women who are pregnant with significant heart disease, congenital or acquired.

Medical Exemptions continued • People with specific cancers: § § § people with cancer who are undergoing active chemotherapy or radical radiotherapy for lung cancer people with cancers of the blood or bone marrow such as leukaemia, lymphoma or myeloma who are at any stage of treatment people having immunotherapy or other continuing antibody treatments for cancer people having other targeted cancer treatments which can affect the immune system, such as protein kinase inhibitors or PARP inhibitors people who have had bone marrow or stem cell transplants in the last 6 months, or who are still taking immunosuppression drugs

The Purpose of Fit Testing is used to assess whether a specific make, model and size of tightfitting respirator is able to provide an adequate fit to an individual wearer. • The performance of tight-fitting facepieces depends on achieving a good contact between the wearer’s skin and the face seal of the facepiece. • People’s faces vary significantly in shape and size so it is unlikely that one particular type or size will fit everyone. • Inadequate fit will significantly reduce the protection provided to the wearer, endangering the wearer’s life or health. • Fit Testing is useful to check that the wearer knows how to correctly don and wear a specific respirator without assistance.

When to use Filtering Face Piece (class 3) (FFP 3) respirators

Aerosol Generating Procedures The following procedures are currently considered to be potentially infectious AGPs for COVID-19: • Intubation, extubation and related procedures, for example, manual ventilation and open suctioning of the respiratory tract (including the upper respiratory tract) • Tracheotomy or tracheostomy procedures (insertion or open suctioning or removal) • Bronchoscopy and upper ENT airway procedures that involve suctioning • Upper gastro-intestinal endoscopy where there is open suctioning of the upper respiratory tract • Surgery and post mortem procedures involving high-speed devices • Some dental procedures (for example, high-speed drilling) • Non-invasive ventilation (NIV); Bi-level Positive Airway Pressure Ventilation (Bi. PAP) and Continuous Positive Airway Pressure Ventilation (CPAP) • High Frequency Oscillatory Ventilation (HFOV) • Induction of sputum • High flow nasal oxygen (HFNO)

Resuscitation The GMMH trust are aware that there is conflicting guidance from the Resus Council and PHE. However, the position being taken by GMMH approved by Gold Command is to follow the Resus Council guidance. Please ignore the PHE resus guidance. The situation is under review Nationally Resus Council Guidance Identify as early as possible any patients who are at risk of acute deterioration or cardiac arrest. Take appropriate steps to prevent cardiac arrest and avoid unprotected CPR. Use of physiological track-and-trigger systems (e. g. NEWS 2) will enable early detection of acutely ill patients. For those for whom resuscitation would be inappropriate, decisions must be made and communicated. Consider discussion with acute hospitals on the need to transfer the patient for acute/advanced medical care.

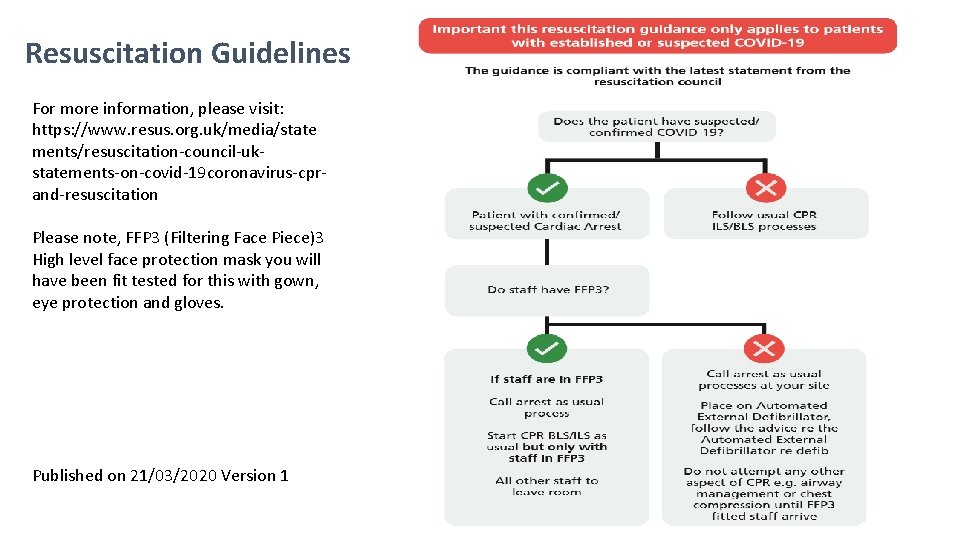
Resuscitation Guidelines For more information, please visit: https: //www. resus. org. uk/media/state ments/resuscitation-council-ukstatements-on-covid-19 coronavirus-cprand-resuscitation Please note, FFP 3 (Filtering Face Piece)3 High level face protection mask you will have been fit tested for this with gown, eye protection and gloves. Published on 21/03/2020 Version 1

Resuscitation of COVID-19 patients in non- acute hospital settings 1 Recognise cardiac arrest. Look for the absence of signs of life and normal breathing. Feel for a carotid pulse if trained to do so. Do not listen or feel for breathing by placing your ear and cheek close to the patient’s mouth. When calling 999/2222, state the risk of COVID 19. 2 If a defibrillator is readily available defibrillate shockable rhythms rapidly prior to starting chest compressions. The early restoration of circulation may prevent the need for further resuscitation measures. Local guidance must be followed about equipment entering the area. 3 Level 3 Personal Protective Equipment (PPE) must be worn by all members of the resuscitation/emergency team before entering the room. Sets of Level 3 PPE must be readily available where resuscitation equipment is being locally stored. No chest compressions or airway procedures such as those detailed below should be undertaken without Level 3 PPE. Once suitably clothed, start compression-only CPR and monitor the patient’s cardiac arrest rhythm as soon as possible. Do not do mouth-to-mouth ventilation or use a pocket mask. If the patient is already receiving supplemental oxygen therapy using a face mask, leave the mask on the patient’s face during chest compressions as this may limit aerosol spread. If not in situ, but one is readily available, put a simple oxygen mask on the patient’s face. Restrict the number of staff in the room (if a single room). Allocate a gatekeeper to do this. 4 Airway interventions (e. g. supraglottic airway (SGA) insertion or tracheal intubation) must be carried out by experienced individuals. Individuals should use only the airway skills (e. g. bag-mask ventilation) for which they have received training. For many HCWs this will mean two-person bag-mask techniques with the use of an oropharyngeal airway. Tracheal intubation or SGA insertion must only be attempted by individuals who are experienced and competent in this procedure.

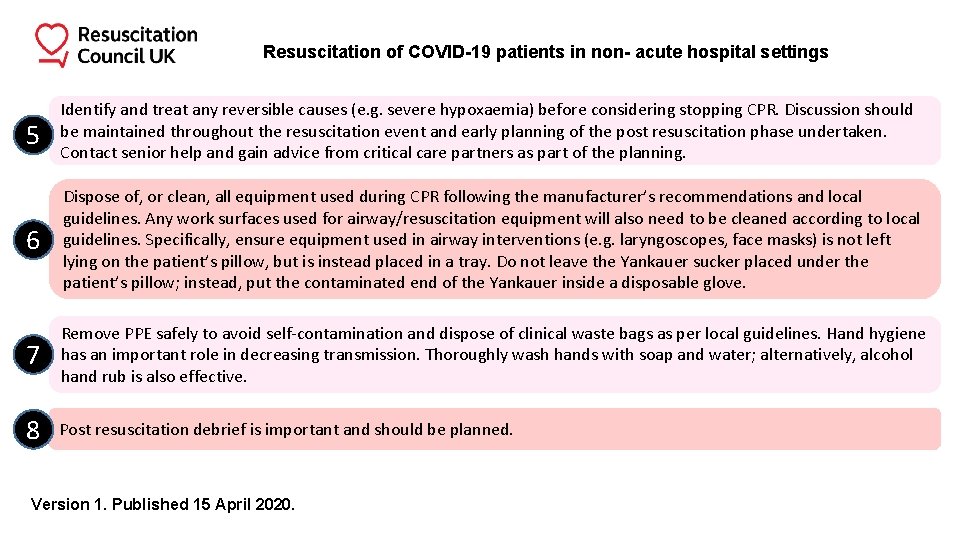
Resuscitation of COVID-19 patients in non- acute hospital settings 5 Identify and treat any reversible causes (e. g. severe hypoxaemia) before considering stopping CPR. Discussion should be maintained throughout the resuscitation event and early planning of the post resuscitation phase undertaken. Contact senior help and gain advice from critical care partners as part of the planning. 6 Dispose of, or clean, all equipment used during CPR following the manufacturer’s recommendations and local guidelines. Any work surfaces used for airway/resuscitation equipment will also need to be cleaned according to local guidelines. Specifically, ensure equipment used in airway interventions (e. g. laryngoscopes, face masks) is not left lying on the patient’s pillow, but is instead placed in a tray. Do not leave the Yankauer sucker placed under the patient’s pillow; instead, put the contaminated end of the Yankauer inside a disposable glove. 7 Remove PPE safely to avoid self-contamination and dispose of clinical waste bags as per local guidelines. Hand hygiene has an important role in decreasing transmission. Thoroughly wash hands with soap and water; alternatively, alcohol hand rub is also effective. 8 Post resuscitation debrief is important and should be planned. Version 1. Published 15 April 2020.

Filtering Face Piece (class 3) (FFP 3) respirators Respirators are used to prevent inhalation of small airborne particles arising from AGPs. All respirators should: • be well fitted, covering both nose and mouth • not be allowed to dangle around the neck of the wearer after or between each use • not be touched once put on • be removed outside the patient room or cohort area or COVID-19 ward Respirators can be single use or single session use (disposable) and fluid-resistant. Note that valved respirators are not fully fluid-resistant unless they are also ‘shrouded’. Valved, non-shrouded FFP 3 respirators are not considered to be fluid resistant and therefore should be worn with a full face shield if blood or body fluid splashing is anticipated.

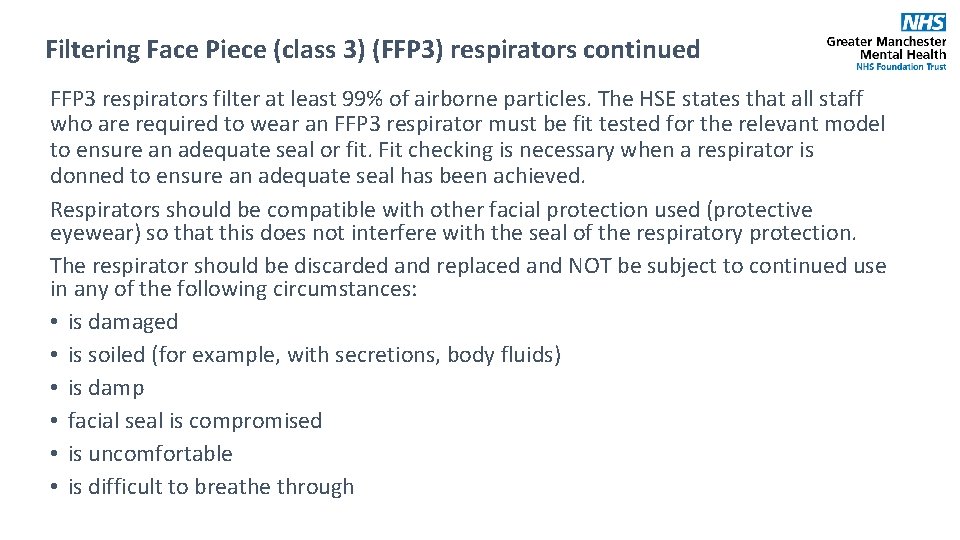
Filtering Face Piece (class 3) (FFP 3) respirators continued FFP 3 respirators filter at least 99% of airborne particles. The HSE states that all staff who are required to wear an FFP 3 respirator must be fit tested for the relevant model to ensure an adequate seal or fit. Fit checking is necessary when a respirator is donned to ensure an adequate seal has been achieved. Respirators should be compatible with other facial protection used (protective eyewear) so that this does not interfere with the seal of the respiratory protection. The respirator should be discarded and replaced and NOT be subject to continued use in any of the following circumstances: • is damaged • is soiled (for example, with secretions, body fluids) • is damp • facial seal is compromised • is uncomfortable • is difficult to breathe through

Other PPE Required Donning & Doffing

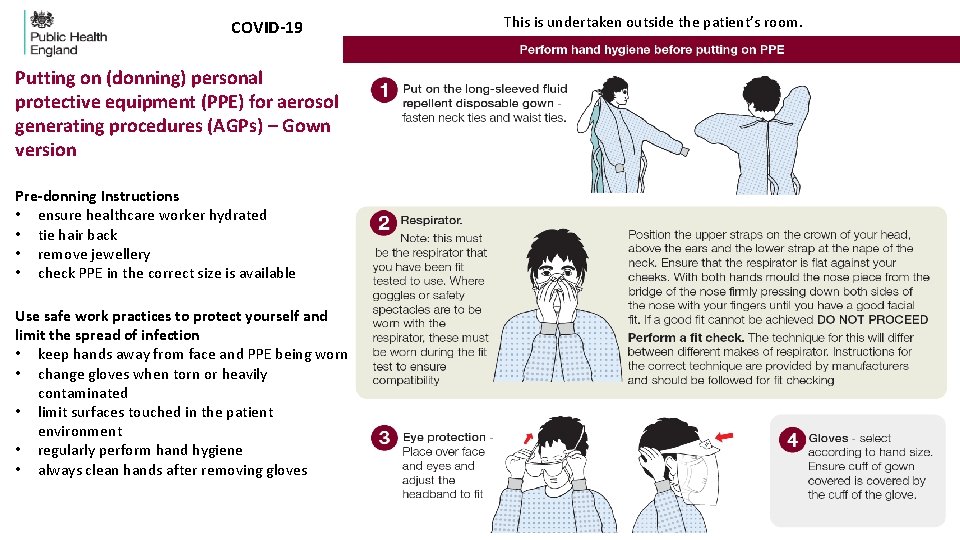
COVID-19 Putting on (donning) personal protective equipment (PPE) for aerosol generating procedures (AGPs) – Gown version Pre-donning Instructions • ensure healthcare worker hydrated • tie hair back • remove jewellery • check PPE in the correct size is available Use safe work practices to protect yourself and limit the spread of infection • keep hands away from face and PPE being worn • change gloves when torn or heavily contaminated • limit surfaces touched in the patient environment • regularly perform hand hygiene • always clean hands after removing gloves This is undertaken outside the patient’s room.

Putting on (donning) personal protective equipment (PPE) for aerosol generated procedures (AGPs)

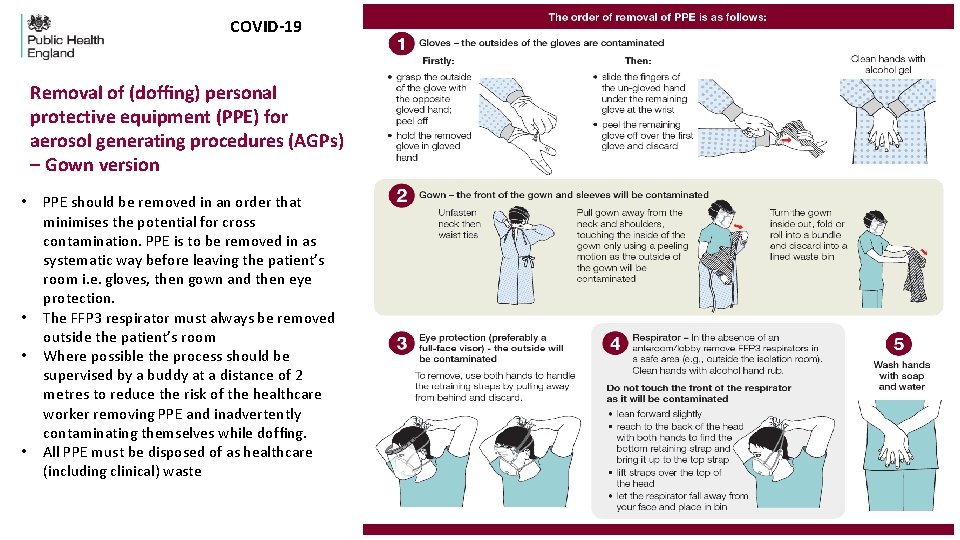
COVID-19 Removal of (doffing) personal protective equipment (PPE) for aerosol generating procedures (AGPs) – Gown version • • PPE should be removed in an order that minimises the potential for cross contamination. PPE is to be removed in as systematic way before leaving the patient’s room i. e. gloves, then gown and then eye protection. The FFP 3 respirator must always be removed outside the patient’s room Where possible the process should be supervised by a buddy at a distance of 2 metres to reduce the risk of the healthcare worker removing PPE and inadvertently contaminating themselves while doffing. All PPE must be disposed of as healthcare (including clinical) waste

Removal of (doffing) personal protective equipment (PPE) for aerosol generated procedures (AGPs)

The Qualitative Fit Testing Process

Pre-testing Factors Wearer Factors Facial Hair - For tight-fitting RPE, the wearer must be clean shaven in the region of the face seal. Smoking, Eating & Drinking - The wearer should not smoke, vape, eat or drink for at least 30 minutes prior to the fit test and must have a clean palate to conduct the sensitivity and fit test. Other PPE - The wearer should wear during the fit test any other PPE normally used in the workplace which may interfere with the fit of the facepiece, eg: safety glasses / goggles.

The Sensitivity Test This part of the test is conducted WITHOUT a respirator mask The Sensitivity Solution is a very dilute version of the Fit Test Solution and is used to assess whether the wearer is able to taste the solution and to what level. • Determine the number of squeezes (10, 20 or 30) required for taste to be detected by wearer • The wearer must breathe through the mouth with their tongue extended. • If the wearer cannot detect the Sensitivity Solution after a maximum of 30 squeezes, then another type of fit test must be used • After the Sensitivity Test, the wearer must cleanse their palette with plain water and wipe their face to remove any residue before proceeding to the fit test

The Fit Test • Ensure a clean palette after the Sensitivity Test. • Don the mask and other PPE and check for fit (Buddy to offer assistance and perform further checks) • Fit Test Solution will be administered into the hood using the number of squeezes (10, 20 or 30) as determined in the Sensitivity Test • The aerosol concentration in the hood will be maintained by administering half the number of squeezes (5, 10 or 15) every 30 seconds for the duration of the fit test • The 7 test exercises will be performed

The 7 Fit Testing Exercises INDG 479 protocol requires 7 test exercises for all test methods. The purpose is to stress the facepiece and simulate normal workplace movement 60 -seconds for each exercise All 7 exercises must be passed in one test 1. Normal Breathing Breathe normally, no head movements or talking 2. Deep Breathing Breathe slowly and deeply 3. Head Side to Side Turn the head slowly from side to side between extreme positions on each side. Approximately 15– 20 times per minute. Inhale at each extreme 4. Head Up and Down Move the head slowly up and down. Approximately 15 – 20 times per minute. Inhale at top position 5. Talking Talk out loud slowly reading from a prepared text such as the Rainbow passage/count backwards from 100 6. Bending Bend at the waist as if to touch toes 7. Normal Breathing Breathe normally, no head movements or talking

The Fit Test If the wearer does not taste the test solution during the fit test then the fit is deemed adequate and the fit test result is recorded as a ‘PASS’. If the wearer tastes the test solution during the fit test, then the fit is deemed inadequate and the fit test result is recorded as a ‘FAIL’.

Actions Following a FAIL Result • Remove the fit test hood and examine the fit of the mask • Remove the mask, cleanse the palate and wipe the face • Re-inspect the mask to check for faults or damage • Allow at least 30 minutes for the palate to clear (drink only water) • Repeat the WHOLE test (including the sensitivity test AND the fit test) • Repeat test can only be performed once with same make, model and size of mask • Record the fail result and any subsequent pass results

Repeat Fit Testing will be required every 6 months Repeat Fit Testing will be required before the expiry date if: • the make, model or size of facepiece changes • the wearer uses any other PPE or spectacles not worn during the fit test which may interfere with the fit of the facepiece. • the wearer experiences any significant changes to their facial characteristics (e. g. through weight gain/loss, dentistry, scars, moles etc)

Debrief The wearer should be debriefed on the following points: • The overall result of the fit test • Implications of the result • When a repeat fit test is required • Which make, model and size of mask they were fit tested with • Any ongoing training requirements