Ferchmin 2019 GLUCONEOGENESIS Index of slides Index 3







- Slides: 20

Ferchmin 2019 GLUCONEOGENESIS Index of slides Index 3) Description of gluconeogenesis. 4) Comparison between glycolysis and gluconeogenesis. 8 -12) Reversal in gluconeogenesis of pyruvate kinase. 13) Steps shared by glycolysis and gluconeogenesis. 14 -16) Release of free glucose, von Gierke and alcohol mediate hypoglycemia. 17) Cori and alanine cycles. 18 -19) Regulation of gluconeogenesis. 20) Purpose of gluconeogenesis. Glucogenic and not glucogenic metabolites. 1

Objectives or what you ought to learn from this file Outline the gluconeogenesis pathway indicating how it differs from glycolysis in function and mechanism Understand hypoglycemia secondary to ethanol ingestion. Know the role of the mitochondria in gluconeogenesis from pyruvate and lactate. Cori cycle and its function. Understand von Gierke disease 2

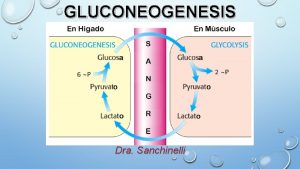
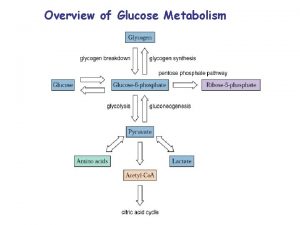
Gluconeogenesis is the synthesis of glucose from precursors that are not sugars, lactate, pyruvate, glycerol or glycogenic amino acids. The synthesis of glucose from other sugars is not gluconeogenesis. The neo means de novo from noncarbohydrate molecules. There is no gluconeogenesis from fatty acids except the rare ones with the odd number of carbons which have a minute contribution to the synthesis of glucose, and its role is of rather academic value. Fatty acids contribute to the fasting organism with ATP through β-oxidation and oxidation of ketone bodies in the Krebs cycle. Ketone bodies only partially substitute for glucose and are synthesized by a pathway different from gluconeogenesis. Ketone bodies are potentially dangerous in the absence of glucose (you will study this later). In conclusion: lipids can spare glucose because they provide for ATP that otherwise would be synthesized in glycolysis. However, lipids do not substitute glucose. We need about l 60 grams of glucose per day, 120 grams are required for the brain and 40 grams for muscle, erythrocytes, eye lens cells, and kidneys medulla. Approximately 200 grams are stored in hepatic glycogen. Gluconeogenesis provides the necessary glucose during fast. The complete gluconeogenesis occurs in the liver and a small fraction in the kidney. Since glycolysis is irreversible gluconeogenesis cannot be the reversal of glycolysis. The enzymes that catalyze the irreversible reactions in glycolysis are overridden in various ingenious ways in gluconeogenesis. 3

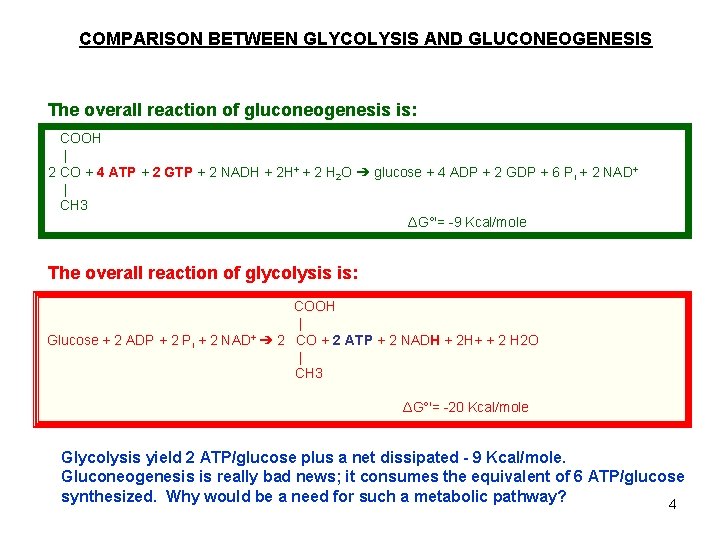
COMPARISON BETWEEN GLYCOLYSIS AND GLUCONEOGENESIS The overall reaction of gluconeogenesis is: COOH | 2 CO + 4 ATP + 2 GTP + 2 NADH + 2 H+ + 2 H 2 O ➔ glucose + 4 ADP + 2 GDP + 6 Pi + 2 NAD+ | CH 3 ΔG°'= -9 Kcal/mole The overall reaction of glycolysis is: COOH | Glucose + 2 ADP + 2 Pi + 2 NAD+ ➔ 2 CO + 2 ATP + 2 NADH + 2 H+ + 2 H 2 O | CH 3 ΔG°'= -20 Kcal/mole Glycolysis yield 2 ATP/glucose plus a net dissipated - 9 Kcal/mole. Gluconeogenesis is really bad news; it consumes the equivalent of 6 ATP/glucose synthesized. Why would be a need for such a metabolic pathway? 4

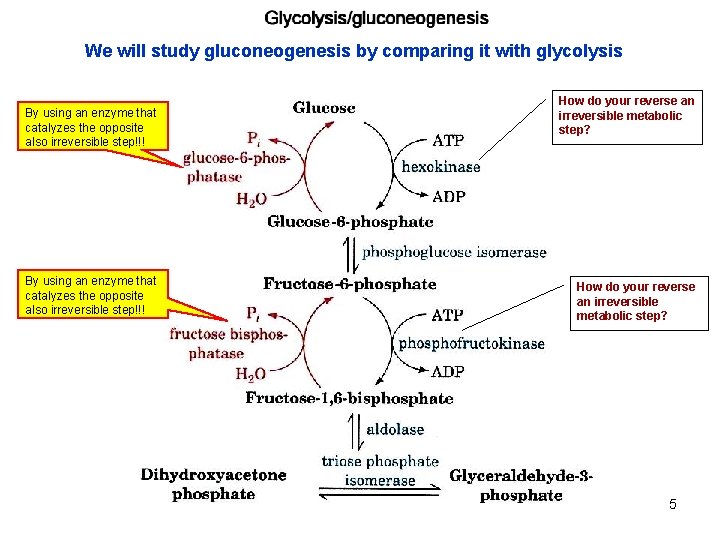
We will study gluconeogenesis by comparing it with glycolysis By using an enzyme that catalyzes the opposite also irreversible step!!! How do your reverse an irreversible metabolic step? 5

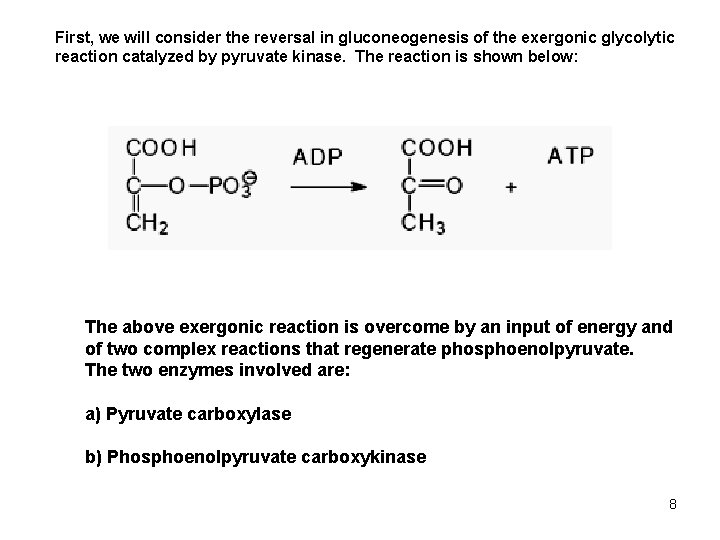
The last glycolytic step catalyzed by pyruvate kinase is irreversible, the free energy change is high, -7. 5 Kcal/mole. To reverse this step in gluconeogenesis two enzymes are used and the process takes place in two cellular compartments. 6

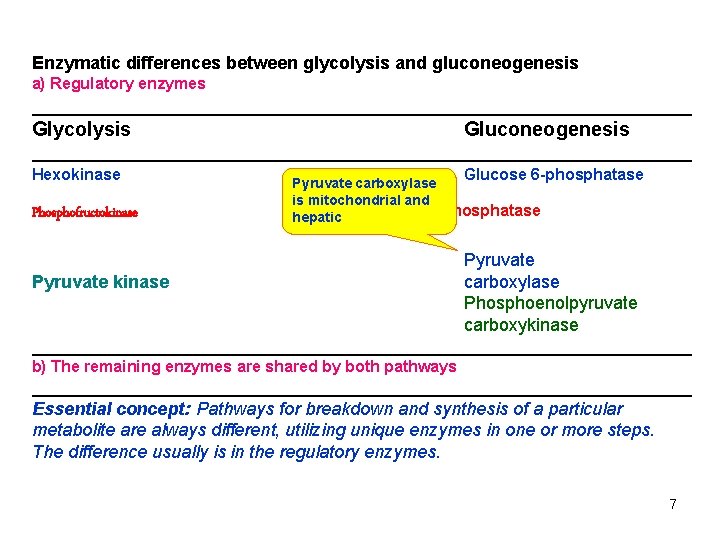
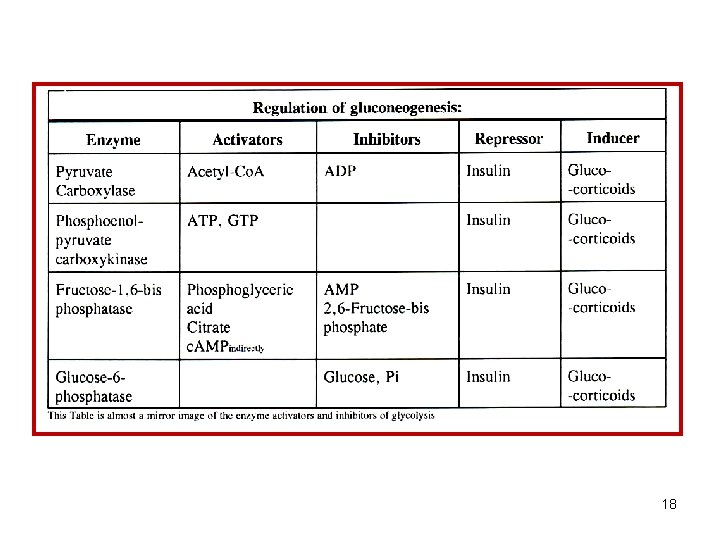
Enzymatic differences between glycolysis and gluconeogenesis a) Regulatory enzymes _________________________________ Glycolysis Gluconeogenesis _________________________________ Hexokinase Glucose 6 -phosphatase Pyruvate carboxylase Phosphofructokinase is mitochondrial and Fructose 1, 6 -bisphosphatase hepatic Pyruvate kinase Pyruvate carboxylase Phosphoenolpyruvate carboxykinase _________________________________ b) The remaining enzymes are shared by both pathways _________________________________ Essential concept: Pathways for breakdown and synthesis of a particular metabolite are always different, utilizing unique enzymes in one or more steps. The difference usually is in the regulatory enzymes. 7

First, we will consider the reversal in gluconeogenesis of the exergonic glycolytic reaction catalyzed by pyruvate kinase. The reaction is shown below: The above exergonic reaction is overcome by an input of energy and of two complex reactions that regenerate phosphoenolpyruvate. The two enzymes involved are: a) Pyruvate carboxylase b) Phosphoenolpyruvate carboxykinase 8

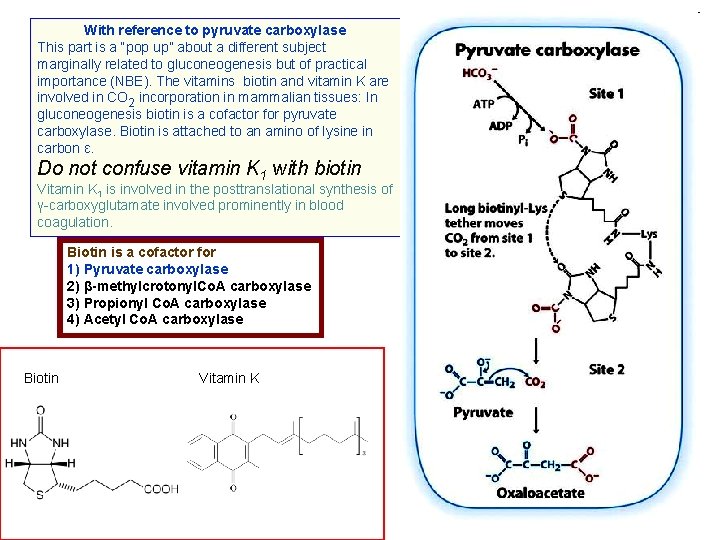
With reference to pyruvate carboxylase This part is a “pop up” about a different subject marginally related to gluconeogenesis but of practical importance (NBE). The vitamins biotin and vitamin K are involved in CO 2 incorporation in mammalian tissues: In gluconeogenesis biotin is a cofactor for pyruvate carboxylase. Biotin is attached to an amino of lysine in carbon ε. Do not confuse vitamin K 1 with biotin Vitamin K 1 is involved in the posttranslational synthesis of γ-carboxyglutamate involved prominently in blood coagulation. Biotin is a cofactor for 1) Pyruvate carboxylase 2) β-methylcrotonyl. Co. A carboxylase 3) Propionyl Co. A carboxylase 4) Acetyl Co. A carboxylase Biotin Vitamin K 9

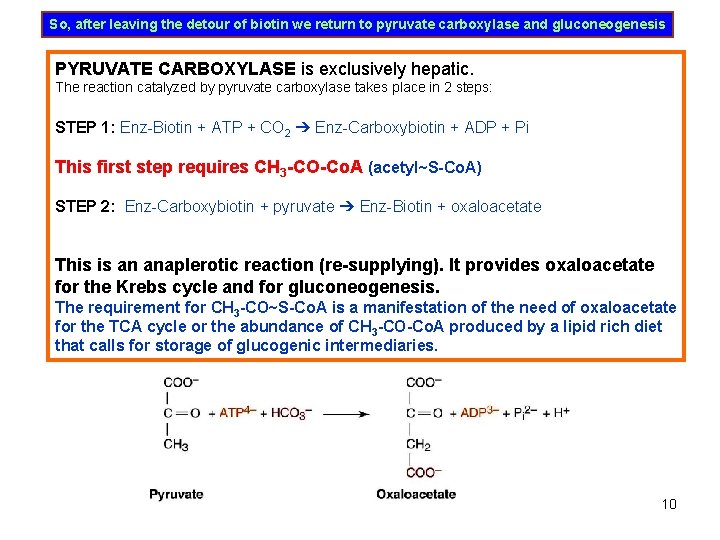
So, after leaving the detour of biotin we return to pyruvate carboxylase and gluconeogenesis PYRUVATE CARBOXYLASE is exclusively hepatic. The reaction catalyzed by pyruvate carboxylase takes place in 2 steps: STEP 1: Enz-Biotin + ATP + CO 2 ➔ Enz-Carboxybiotin + ADP + Pi This first step requires CH 3 -CO-Co. A (acetyl~S-Co. A) STEP 2: Enz-Carboxybiotin + pyruvate ➔ Enz-Biotin + oxaloacetate This is an anaplerotic reaction (re-supplying). It provides oxaloacetate for the Krebs cycle and for gluconeogenesis. The requirement for CH 3 -CO~S-Co. A is a manifestation of the need of oxaloacetate for the TCA cycle or the abundance of CH 3 -CO-Co. A produced by a lipid rich diet that calls for storage of glucogenic intermediaries. 10

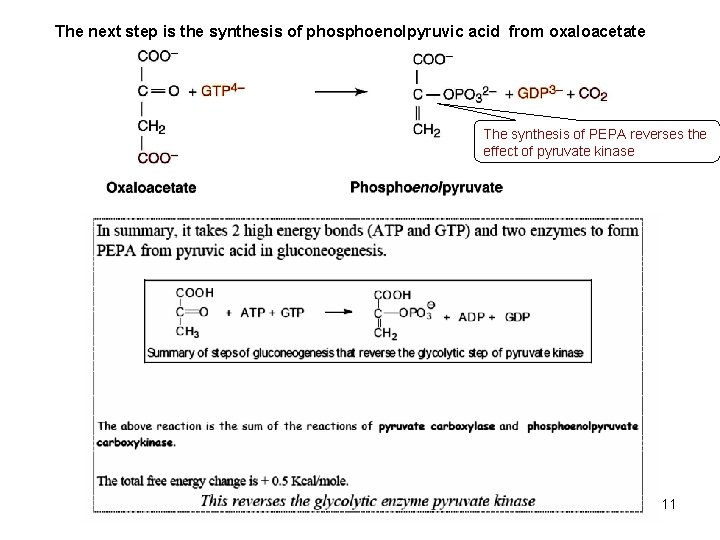
The next step is the synthesis of phosphoenolpyruvic acid from oxaloacetate The synthesis of PEPA reverses the effect of pyruvate kinase 11

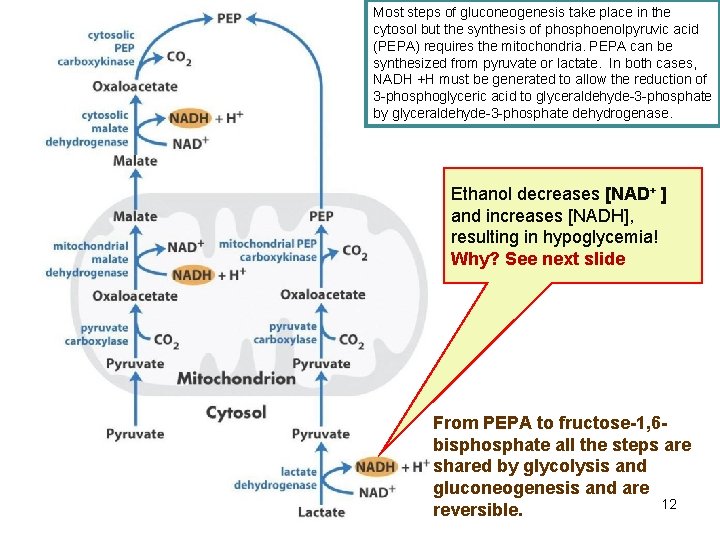
Most steps of gluconeogenesis take place in the cytosol but the synthesis of phosphoenolpyruvic acid (PEPA) requires the mitochondria. PEPA can be synthesized from pyruvate or lactate. In both cases, NADH +H must be generated to allow the reduction of 3 -phosphoglyceric acid to glyceraldehyde-3 -phosphate by glyceraldehyde-3 -phosphate dehydrogenase. Ethanol decreases [NAD+ ] and increases [NADH], resulting in hypoglycemia! Why? See next slide From PEPA to fructose-1, 6 bisphosphate all the steps are shared by glycolysis and gluconeogenesis and are 12 reversible.

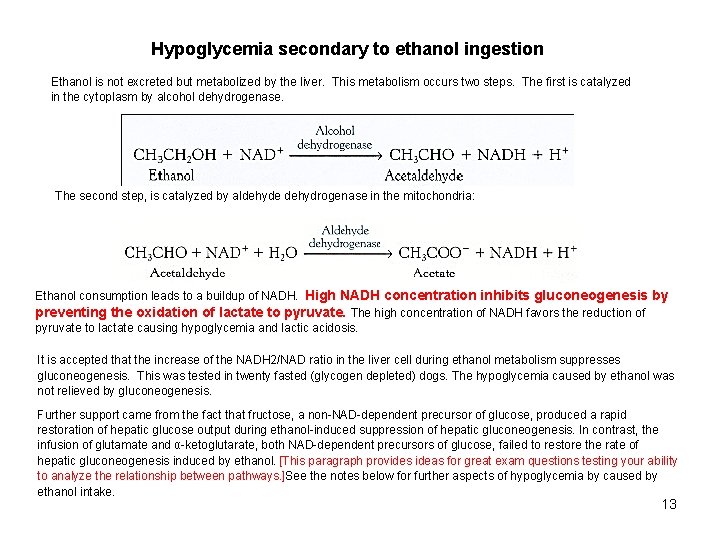
Hypoglycemia secondary to ethanol ingestion Ethanol is not excreted but metabolized by the liver. This metabolism occurs two steps. The first is catalyzed in the cytoplasm by alcohol dehydrogenase. The second step, is catalyzed by aldehyde dehydrogenase in the mitochondria: Ethanol consumption leads to a buildup of NADH. High NADH concentration inhibits gluconeogenesis by preventing the oxidation of lactate to pyruvate. The high concentration of NADH favors the reduction of pyruvate to lactate causing hypoglycemia and lactic acidosis. It is accepted that the increase of the NADH 2/NAD ratio in the liver cell during ethanol metabolism suppresses gluconeogenesis. This was tested in twenty fasted (glycogen depleted) dogs. The hypoglycemia caused by ethanol was not relieved by gluconeogenesis. Further support came from the fact that fructose, a non-NAD-dependent precursor of glucose, produced a rapid restoration of hepatic glucose output during ethanol-induced suppression of hepatic gluconeogenesis. In contrast, the infusion of glutamate and α-ketoglutarate, both NAD-dependent precursors of glucose, failed to restore the rate of hepatic gluconeogenesis induced by ethanol. [This paragraph provides ideas for great exam questions testing your ability to analyze the relationship between pathways. ]See the notes below for further aspects of hypoglycemia by caused by ethanol intake. 13

This graph represents the relationship between the activity of both enzymes and the energy status of a muscle cell. 14

15

von Gierke Glycogen storage disease type I (GSD I), is the most common of the glycogen storage diseases. This genetic disease results from the deficiency of the enzyme glucose-6 -phosphatase. The deficiency does not impair glycogenolysis or glycogen synthesis but impairs the ability of the liver to produce free glucose from glycogen and gluconeogenesis. Therefore, this deficiency causes severe hypoglycemia and results in the accumulation of glycogen in the liver and kidneys. You will have to be able to explain this outcome. Besides hypoglycemia, the direct metabolic derangements in von Gierke patients include lactic acidosis and hyperlipidemia. You will later learn the origin of lactic acidosis and hyperlipidemia. Frequent feedings of cornstarch, raw oats flakes, or other slowly digestible carbohydrates are the primary care to sustain the patient. Otherapeutic measures may be needed for health problems arising later. Glucose-6 -phosphatase is an enzyme located on the inner membrane of the endoplasmic reticulum. The catalytic unit is associated with a calcium-binding protein and three transport proteins that facilitate the movement of glucose-6 -phosphate (G 6 P), phosphate, and glucose (respectively) into and out of the enzyme. This genetic disease has an incidence in the American population of 1 in 50, 000 to 100, 000 births. 16

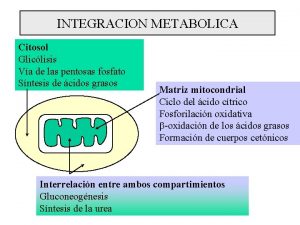
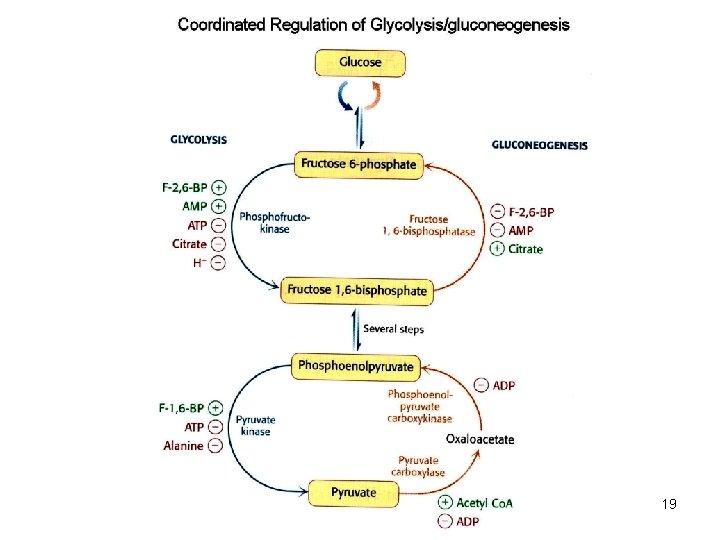
Integration of gluconeogenesis and glycolysis 17

18

19

There is a fundamental difference between the role of glycolysis in the “peripheral” organs and the liver. In the muscle the role of glycolysis is to make ATP but, in the liver, it is to provide pyruvate for the Krebs cycle. Pyruvate is a precursors for the biosynthesis of porphyrins, certain amino acids, and fatty acids. Galactose, fructose, and other monosaccharides are not glucogenic. They are monosaccharides in equilibrium with glucose! Ethanol and fatty acids are not glucogenic (odd number fatty acids contribute insignificantly to gluconeogenesis). Lactate, pyruvate, glycerol, ketoacids of most amino acids, are glucogenic. 20
 A small child slides down the four frictionless slides
A small child slides down the four frictionless slides Energy conservation quick check
Energy conservation quick check Ofsted new framework 2019 slides
Ofsted new framework 2019 slides Ug teaching slides
Ug teaching slides Non carbohydrate sources gluconeogenesis
Non carbohydrate sources gluconeogenesis Glukoneogenez öncülleri
Glukoneogenez öncülleri Sustratos gluconeogenicos
Sustratos gluconeogenicos Glycogenolysis and gluconeogenesis
Glycogenolysis and gluconeogenesis Malate aspartate shuttle
Malate aspartate shuttle Energetics of gluconeogenesis
Energetics of gluconeogenesis Precursores de gluconeogenesis
Precursores de gluconeogenesis Gluconeogenesis definition
Gluconeogenesis definition Gluco genesis
Gluco genesis Glucolisis en el musculo
Glucolisis en el musculo Glycogen metabolism
Glycogen metabolism Types of glycogen storage disease table
Types of glycogen storage disease table Precursor of gluconeogenesis
Precursor of gluconeogenesis Pyruvate dehydrogenase
Pyruvate dehydrogenase Difference between glycolysis and pentose phosphate pathway
Difference between glycolysis and pentose phosphate pathway Glucose synthesis from non-carbohydrate sources
Glucose synthesis from non-carbohydrate sources Gluconeogenesis purpose
Gluconeogenesis purpose