FEMALE GENITAL PATHOLOGY Department of Anatomical Pathology Faculty











- Slides: 85

FEMALE GENITAL PATHOLOGY Department of Anatomical Pathology Faculty of Medicine GMU Yogyakarta

I. Vulva, vagina - Inflammatory diseases - neoplasma

Sexually Transmitted/Infectious Diseases A. VIRAL HERPES (Herpes simplex II virus) Painful red papules group of vesicles ulcerate Intraepidermal vesicles formed by acantholysis due to baloon degeneration of infected epidermal cells CONDYLOMA ACCUMINATA (HPV) Veneral warts flat or verrucous alteration of squamous epithelia Affects: skin of perianal/perineal; mucosa of vagina, cervix, others Verrucous condyloma with hyperkeratosis, parakeratosis, acanthosis, koilocytosis AIDS

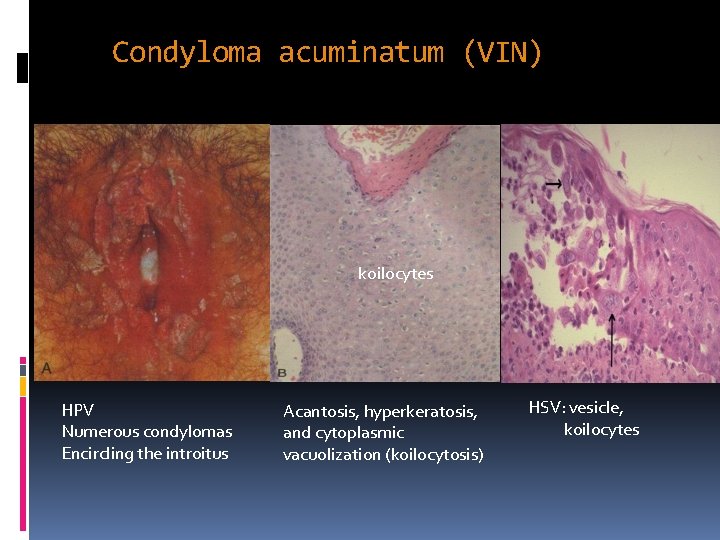
Condyloma acuminatum (VIN) koilocytes HPV Numerous condylomas Encircling the introitus Acantosis, hyperkeratosis, and cytoplasmic vacuolization (koilocytosis) HSV: vesicle, koilocytes

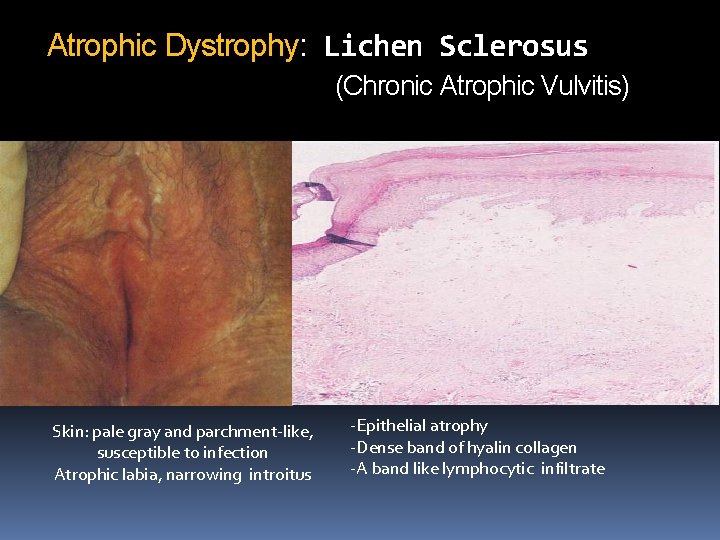
Atrophic Dystrophy: Lichen Sclerosus (Chronic Atrophic Vulvitis) Skin: pale gray and parchment-like, susceptible to infection Atrophic labia, narrowing introitus -Epithelial atrophy -Dense band of hyalin collagen -A band like lymphocytic infiltrate

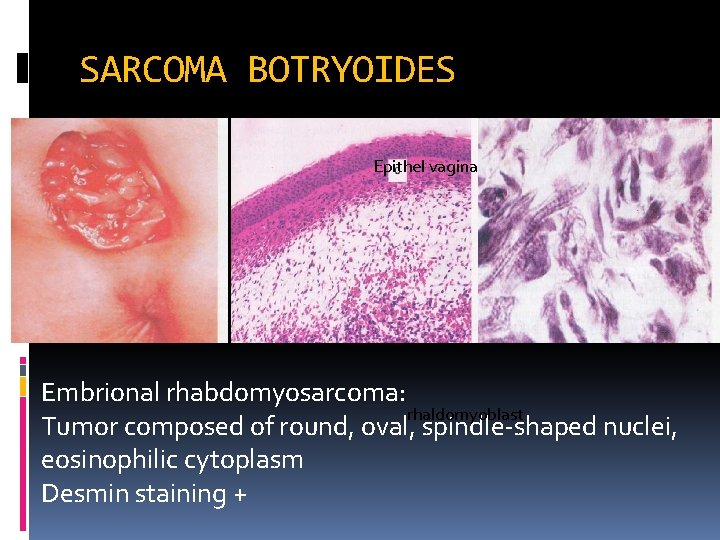
VAGINAL NEOPLASIA Squamous cell carcinoma (SCC) - mostly in fornix posterior, with vaginal discharge - prognosis: 5 -year survival rate : 20 -90% Adenocarsinoma - Arising is the anterior wall of vagina - clear cell carcinoma DES and in young (15 – 27 yo) Sarcoma botryoides - Rhabdomyosarcoma, polypoid grape-like appearance - In general the prognosis is poor

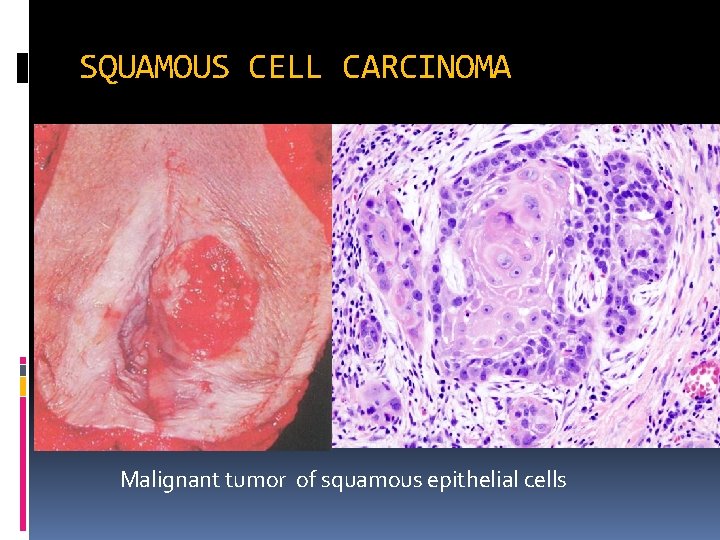
SQUAMOUS CELL CARCINOMA keratinization Malignant tumor of squamous epithelial cells

SARCOMA BOTRYOIDES Epithel vagina Embrional rhabdomyosarcoma: rhaldomyoblast Tumor composed of round, oval, spindle-shaped nuclei, eosinophilic cytoplasm Desmin staining +

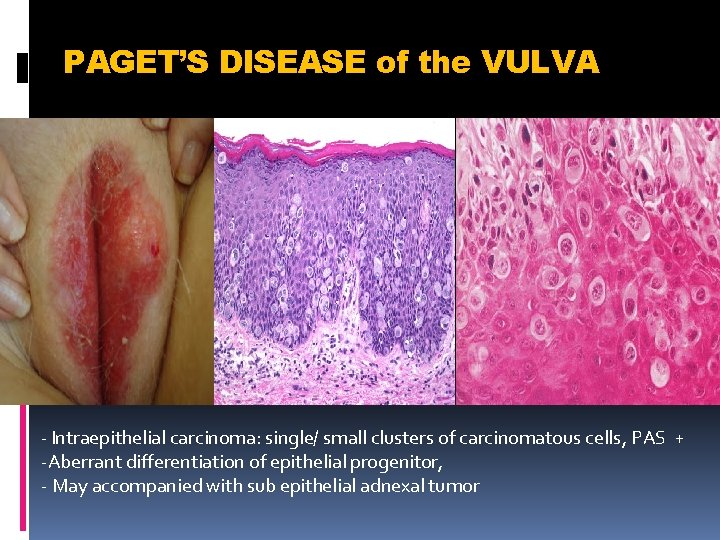
PAGET’S DISEASE of the VULVA - Intraepithelial carcinoma: single/ small clusters of carcinomatous cells, PAS + -Aberrant differentiation of epithelial progenitor, - May accompanied with sub epithelial adnexal tumor

II. Cervix Inflammatory diseases Polyp Carcinoma: squmous cell carcinoma adenocarcinoma

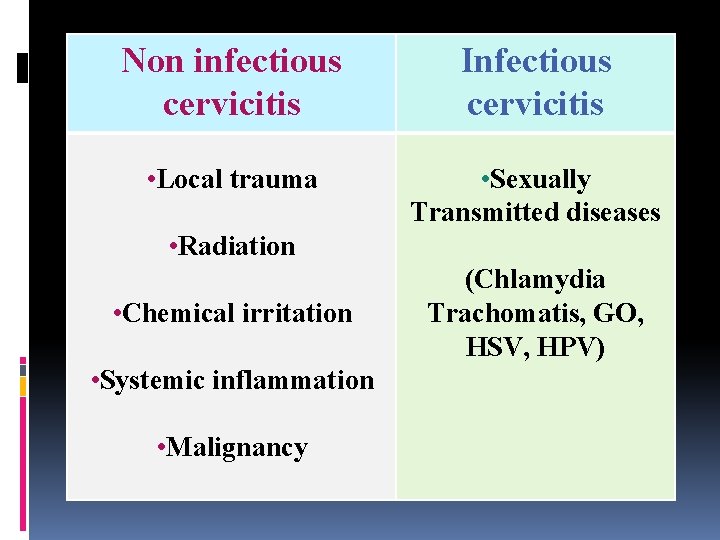
Non infectious cervicitis Infectious cervicitis • Local trauma • Sexually Transmitted diseases • Radiation • Chemical irritation • Systemic inflammation • Malignancy (Chlamydia Trachomatis, GO, HSV, HPV)

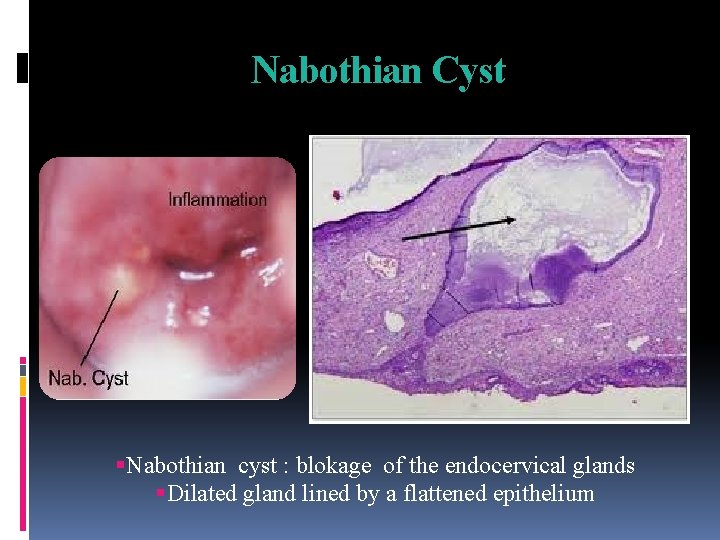
Nabothian Cyst Nabothian cyst : blokage of the endocervical glands Dilated gland lined by a flattened epithelium

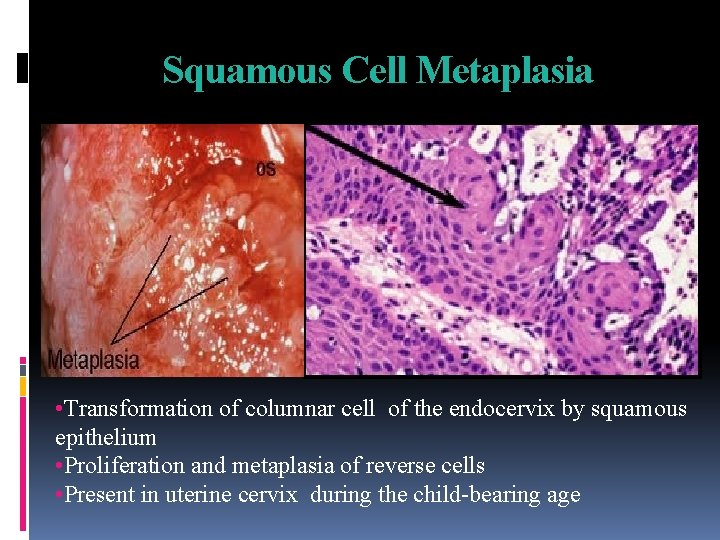
Squamous Cell Metaplasia • Transformation of columnar cell of the endocervix by squamous epithelium • Proliferation and metaplasia of reverse cells • Present in uterine cervix during the child-bearing age

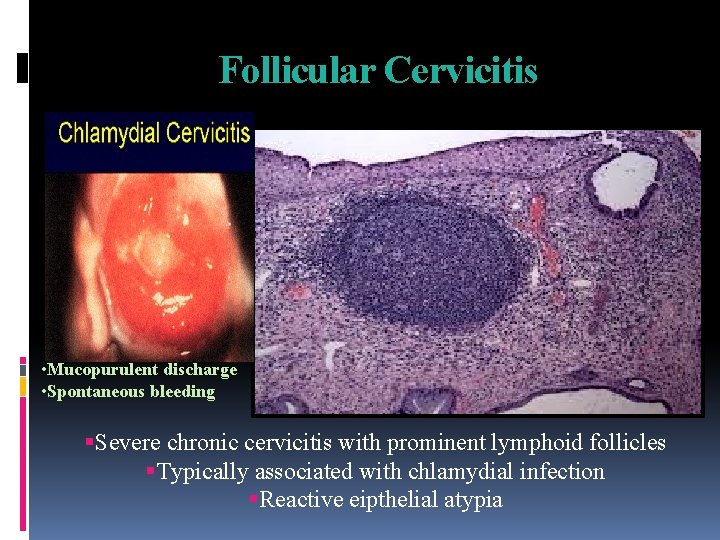
Follicular Cervicitis • Mucopurulent discharge • Spontaneous bleeding Severe chronic cervicitis with prominent lymphoid follicles Typically associated with chlamydial infection Reactive eipthelial atypia

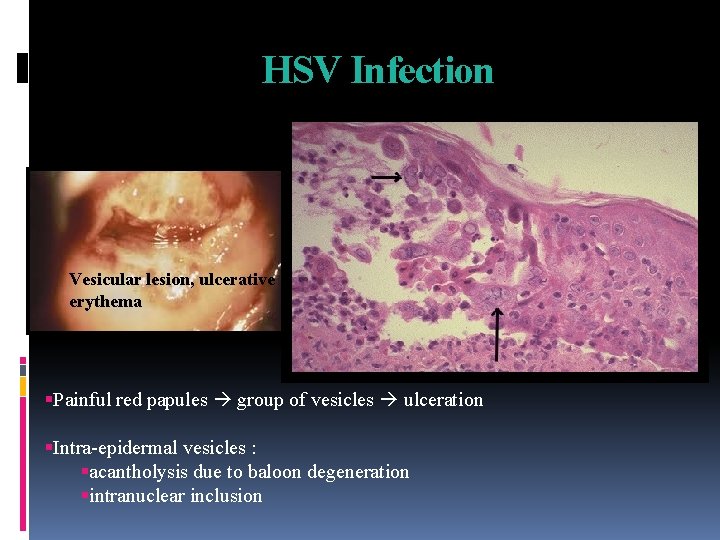
HSV Infection Vesicular lesion, ulcerative erythema Painful red papules group of vesicles ulceration Intra-epidermal vesicles : acantholysis due to baloon degeneration intranuclear inclusion

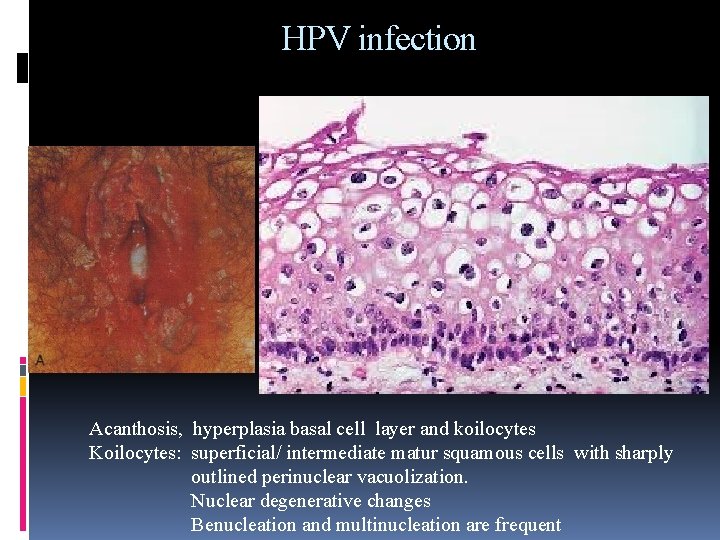
HPV infection Acanthosis, hyperplasia basal cell layer and koilocytes Koilocytes: superficial/ intermediate matur squamous cells with sharply outlined perinuclear vacuolization. Nuclear degenerative changes Benucleation and multinucleation are frequent

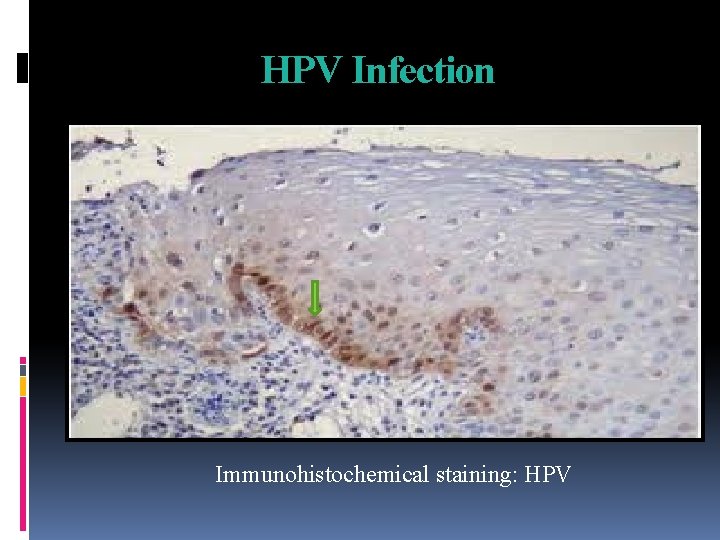
HPV Infection Immunohistochemical staining: HPV

POLYPS Inflammatory 5% of women From endocervical canal – sessile or pedunculated, fibromyxoid Hyperplastic Microglandular hyperplasia consists of tightly pack hypaerplastic endocervical glands

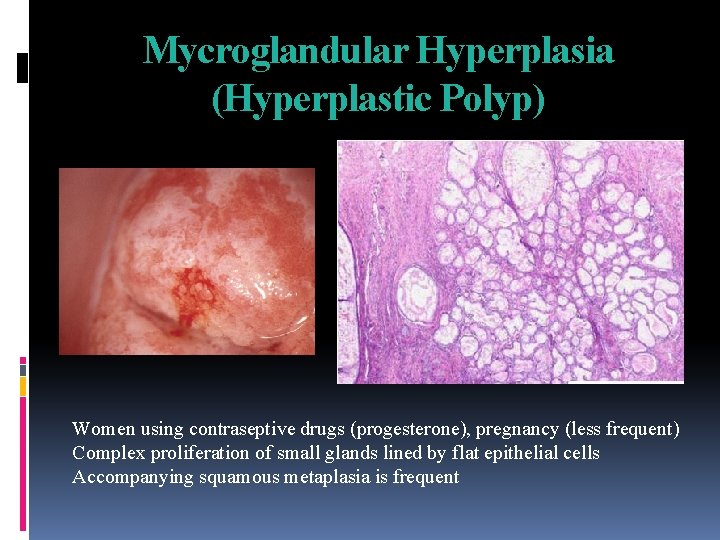
Mycroglandular Hyperplasia (Hyperplastic Polyp) Women using contraseptive drugs (progesterone), pregnancy (less frequent) Complex proliferation of small glands lined by flat epithelial cells Accompanying squamous metaplasia is frequent

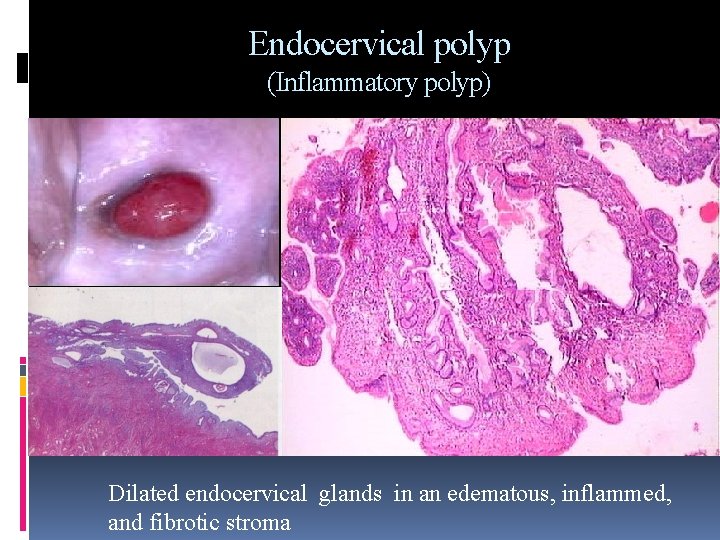
Endocervical polyp (Inflammatory polyp) Dilated endocervical glands in an edematous, inflammed, and fibrotic stroma

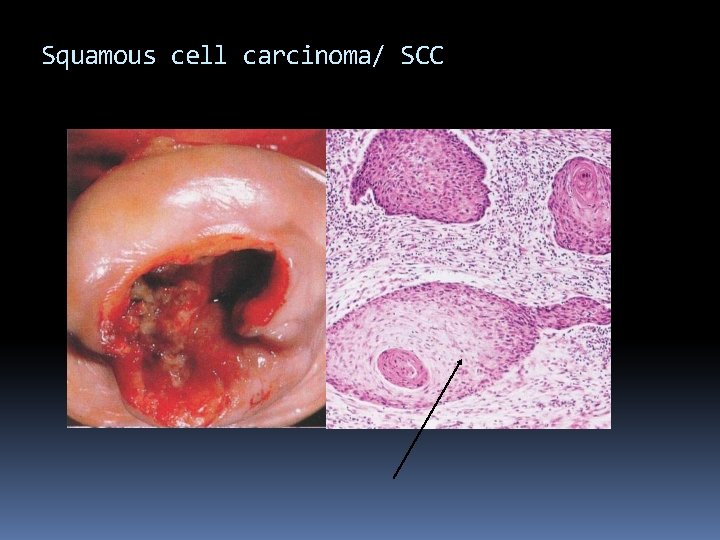
Carcinoma Pathogenesis Location: squamo-collumnar junction Dysplasia (CIN) neoplasia Morphology Grossly: infiltrative, ulcerative, exophytic Microscopic: Squamous cell carcinoma keratinizing, non-keratinizing

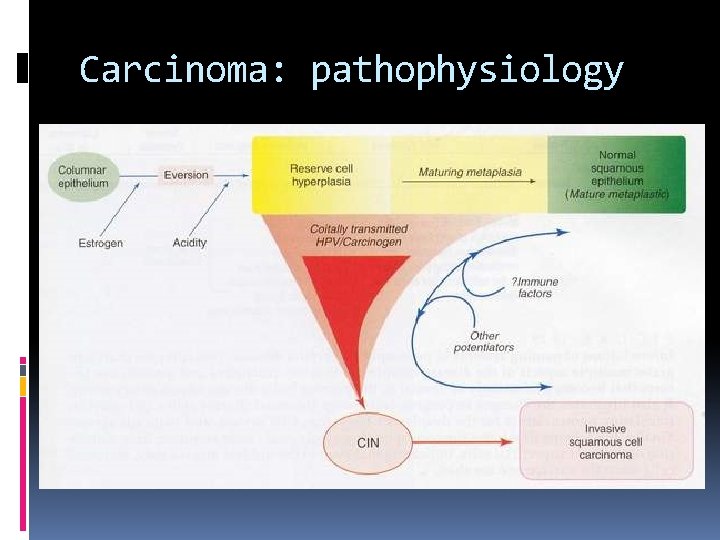
Carcinoma: pathophysiology

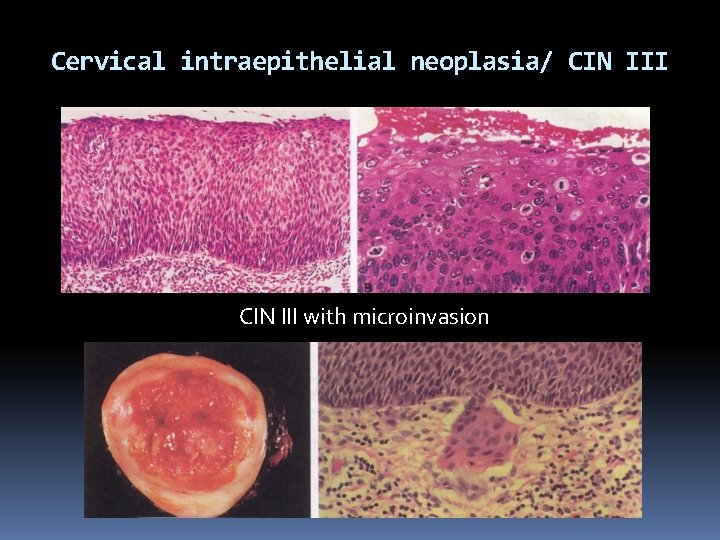
Cervical intraepithelial neoplasia/ CIN III with microinvasion

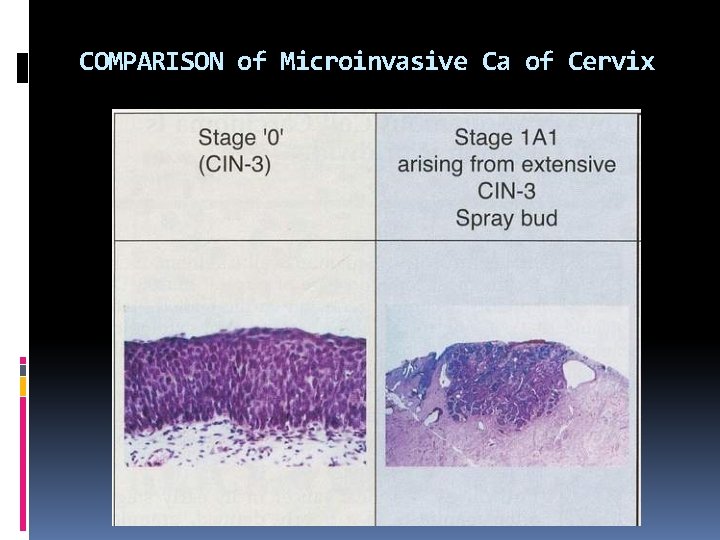
COMPARISON of Microinvasive Ca of Cervix

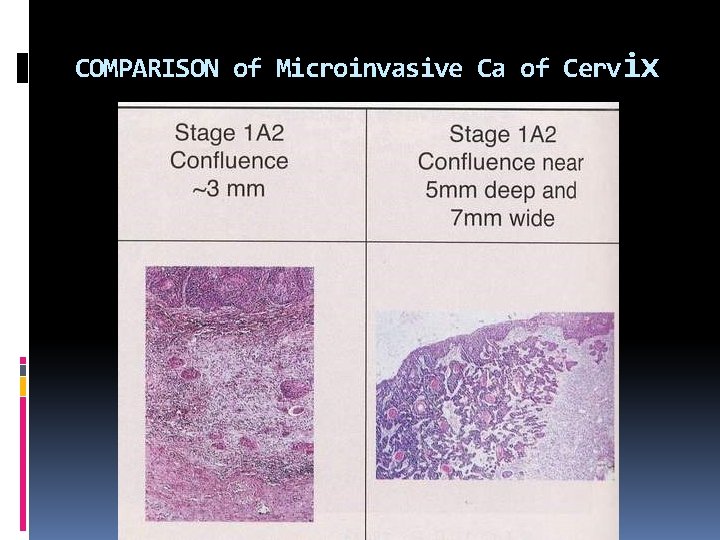
COMPARISON of Microinvasive Ca of Cervix

Squamous cell carcinoma/ SCC

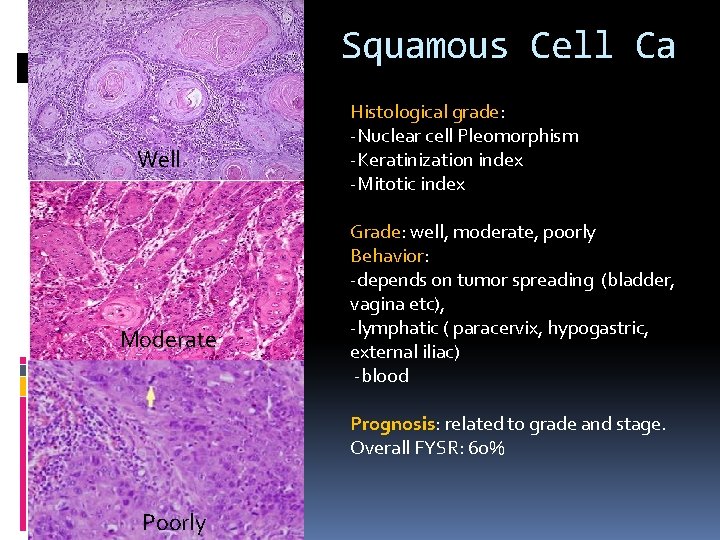
Squamous Cell Ca Well Moderate Histological grade: -Nuclear cell Pleomorphism -Keratinization index -Mitotic index Grade: well, moderate, poorly Behavior: -depends on tumor spreading (bladder, vagina etc), -lymphatic ( paracervix, hypogastric, external iliac) -blood Prognosis: related to grade and stage. Overall FYSR: 60% Poorly

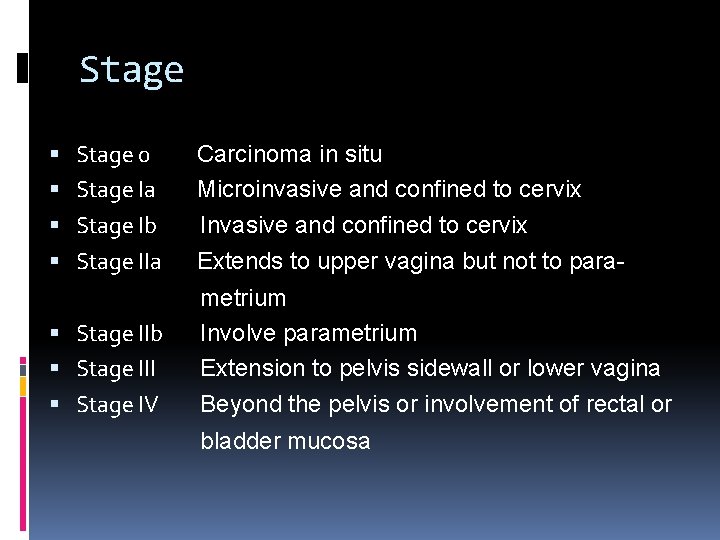
Stage 0 Stage Ia Stage Ib Stage IIa Stage IIb Stage III Stage IV Carcinoma in situ Microinvasive and confined to cervix Invasive and confined to cervix Extends to upper vagina but not to parametrium Involve parametrium Extension to pelvis sidewall or lower vagina Beyond the pelvis or involvement of rectal or bladder mucosa

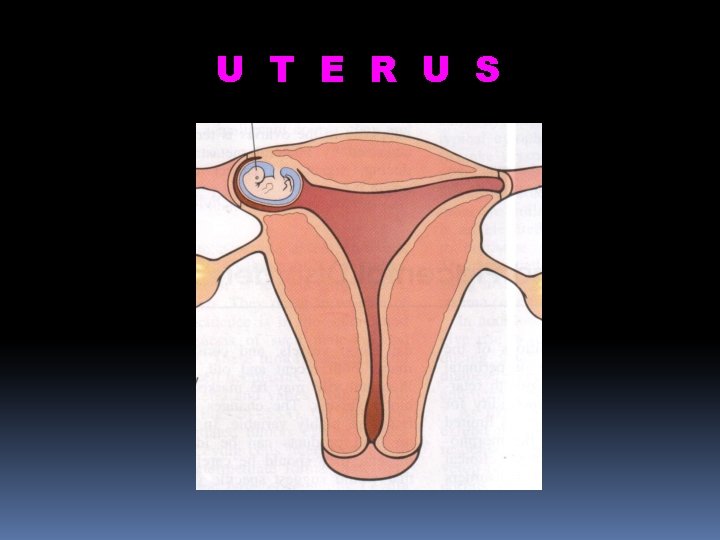
U T E R U S

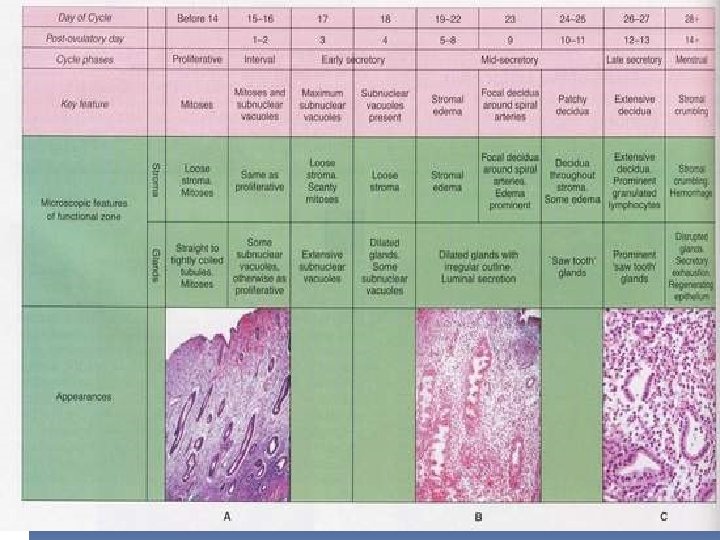
THE MENSTRUAL CYCLE Phases of the endometrium development: Proliferative phase Secretory phase Menstrual phase Atrophic endometrium After the menopause the number of the glands and the quantity stroma progressively decrease The remaining gland are oriented parallel to the surface, the stroma contains abundant collagen The glands are often dilated senile cystic atrophy of the endometrium


PROLIFERATIVE PHASE During the first day of the menstrual cycle the endometrium is under estrogenic stimulation The glands are coiled proliferative progress more coiled pseudostratified columnar watery alkaline secretion facilitate passage of the sperm from endometrial cavity to fallopian tubes

SECRETORY PHASE Ovulation graafian follicle becomes corpus luteum granulosa cells luteinize begin to secrete progesteron transformation of endometrium from proliferative into a secretory state Days 17 -19 (postovulatory days 3 -5) more coiled subnuclear vacuoles scretion to support the zygote Days 20 -22 (postovularory days 6 -8) the endometrium displays prominent glandular secretions and stromal edema. The glands dilate and more tortuous Day 23 (postovulatory day 9) predecidual Day 27 (postovulatory day 13) full thickness of stroma is pre-decidualized and prepared for mentruation. The glands continua to dilate and develop serrated (saw -tooth) border

MENSTRUAL PHASE Absence of pregnancy no blastocyst to elaborate h. CG granulosa & theca cells of the corpus luteum degenerate progesterone level fall - endometrium desiccated - spiral arteries collapse - stroma disintegrates Menses commences on day 28, last 3 or 7 days, and result in a flow of about 35 ml of blood The denuded surface is re-epithelialized by extension of the residual glandular epithelium

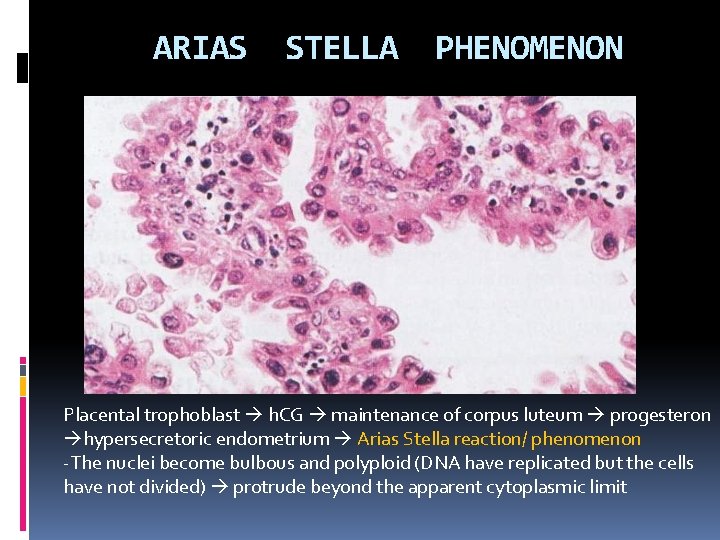
ARIAS STELLA PHENOMENON Placental trophoblast h. CG maintenance of corpus luteum progesteron hypersecretoric endometrium Arias Stella reaction/ phenomenon -The nuclei become bulbous and polyploid (DNA have replicated but the cells have not divided) protrude beyond the apparent cytoplasmic limit

PATHOLOGY OF UTERUS I. ENDOMETRITIS II. ENDOMETRIOSIS III. DYSFUNCTIONAL BLEEDING & ENDOMETRIAL HYPERPLASIA IV. TUMORS OF THE ENDOMETRIUM & MYOMETRIUM

I. ENDOMETRITIS A. Acute endometritis Post-abortion, post-partum states with retaind placental parts Suppurative inflammation + microabscess pyometra obstruction of endocervical canal B. Chronic endometritis Contination of acute endometritis, could be associated with IUD, 15% with unknown etiology Pelvic pain, abnormal bleeding, infertility Infiltration of lymphocytes and plasma cells

II. ENDOMETRIOSIS INTERNAL ENDOMETRIOSIS (ADENOMYOSIS) The presence of endometrial tissue (gland stroma) buried within the myometrium myometrial hypertrophy Thought to arise from abnormal downgrowth of basal endometrium

II. ENDOMETRIOSIS EXTERNAL ENDOMETRIOSIS 20% of adult female (3 rd to 4 th decade) The presence of benign, potentially functional endometrial tissue outside of the uterus significant cause of infertility Pathogenesis: focal differentiation of the coelomic epithelium into endometrial tissue, regurgitation of endometrial tissue outside of the uterus during menses, lymphatic and hematogenous dissemination Clinical: depends on location, generally dysmenorrhea

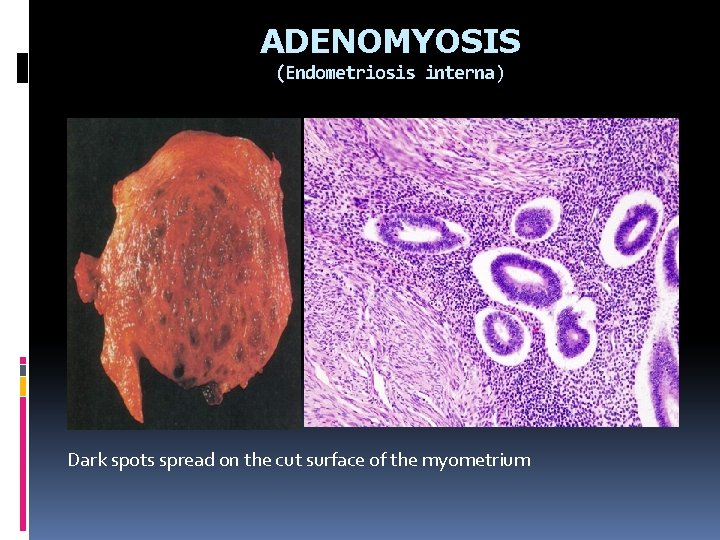
ADENOMYOSIS (Endometriosis interna) Endometrial tissue miometirum Dark spots spread on the cut surface of the myometrium

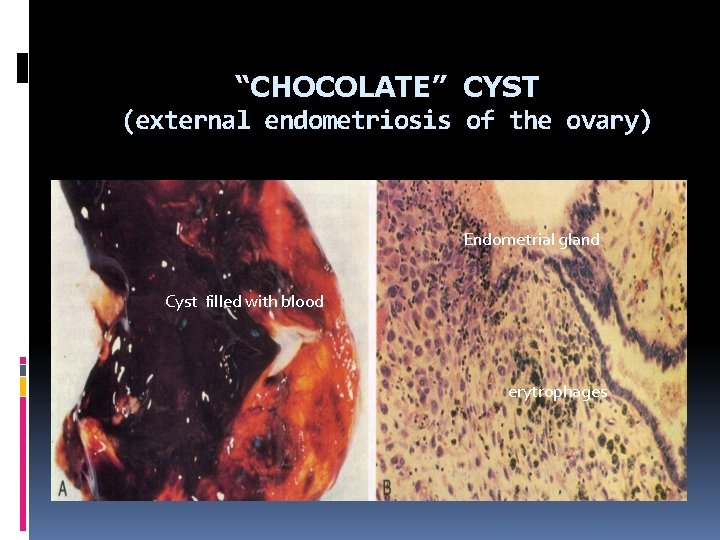
“CHOCOLATE” CYST (external endometriosis of the ovary) Endometrial gland Cyst filled with blood erytrophages

III. DYSFUNCTIONAL UTERINE BLEEDING & ENDOMETRIAL HYPERPLASIA Dysfunctional uterine bleeding Anovulatory cycle Ovulatory cycle - Inadequate luteal phase - Irregular shedding Endometrial hyperplasia Low grade hyperplasia High grade (atypical) hyperplasia

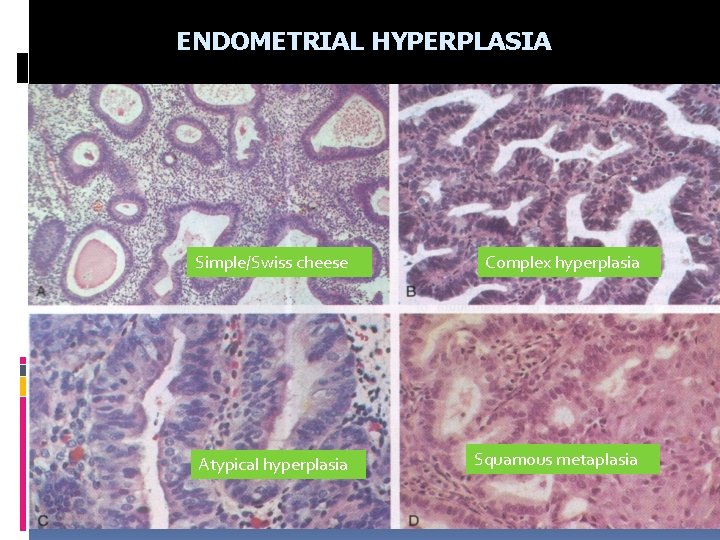
IV ENDOMETRIAL HYPERPLASIA Mostly occur in post-menarchal or peri-menopausal associated to prolonged or excessive estrogen stimulation LOW GRADE HYPERPLASIA Simple low grade hyperplasia Swiss cheese Complex low grade hyperplasia Low to moderate risk of developing carinoma HIGH GRADE (ATYPICAL) HYPERPLASIA High risk to develop carcinoma

ENDOMETRIAL HYPERPLASIA Simple/Swiss cheese Complex hyperplasia Atypical hyperplasia Squamous metaplasia

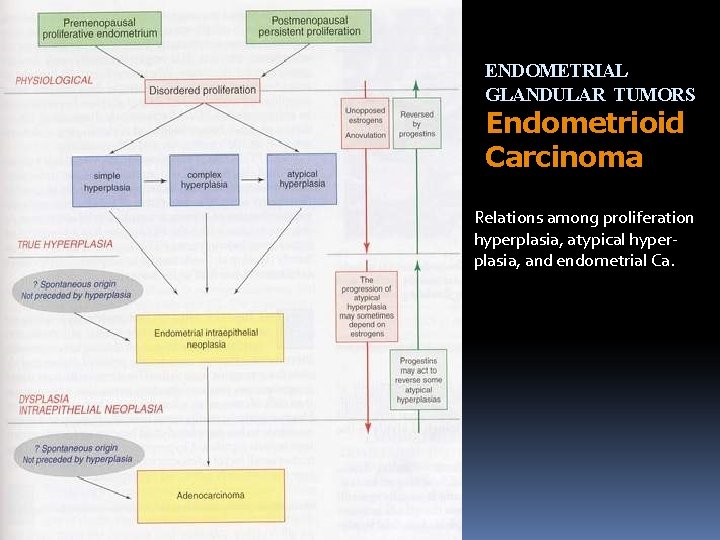
ENDOMETRIAL GLANDULAR TUMORS Endometrioid Carcinoma Relations among proliferation hyperplasia, atypical hyperplasia, and endometrial Ca.

WHO Classification of Tumors of Uterine Corpus Epithelial Tumors and Related Lesions Mesenchymal Tumors Mixed Epithelial and Mesenchymal Tumors Gestational Trophoblastic Disease Miscellaneous Tumors Lymphoid and Hematopoetic Tumors Secondary Tumors

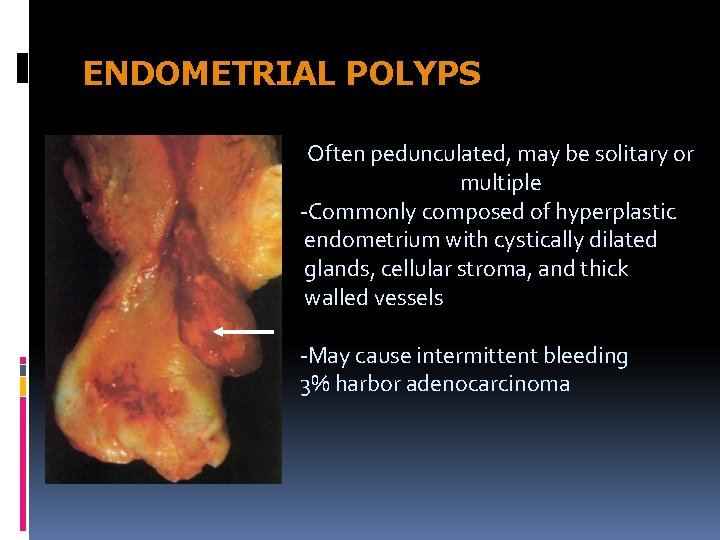
ENDOMETRIAL POLYPS Often pedunculated, may be solitary or multiple -Commonly composed of hyperplastic endometrium with cystically dilated glands, cellular stroma, and thick walled vessels -May cause intermittent bleeding 3% harbor adenocarcinoma

Endometrioid Carcinoma The second most common genital malignancies in Indonesia, and 90% occuring after menopause Principally the development of adenocarcinoma is related to prolonged or excessive estrogen stimulation Risk factors: obesity, diabetes, hypertension, infertility May be associated with functional ovarian tumors, pre -existing hyperplasia, and history of breast cancer Symptoms: irregular vaginal bleeding, leukorrhea

Endometrioid Carcinoma 60% - 75% : G 1 – G 3 20% - 30% : with squamous differentiation adenoacanthoma & adenosquamous carcinoma Prognosis is better predicted by the grade of glandular component Spreading: myometrium adjacent tissue Lymphatic regional and periaortic lymphnode Hematogenous lung, liver, bone, etc. In older women tend to be less-differentiated and more invasive than younger women

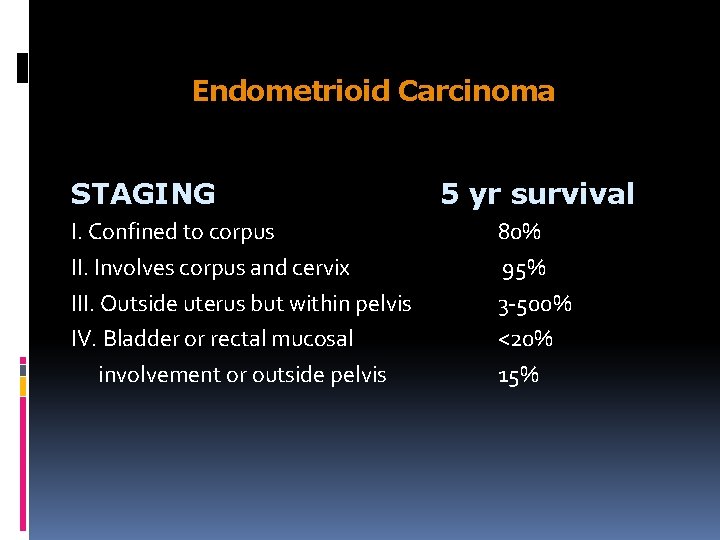
Endometrioid Carcinoma STAGING I. Confined to corpus II. Involves corpus and cervix III. Outside uterus but within pelvis IV. Bladder or rectal mucosal involvement or outside pelvis 5 yr survival 80% 95% 3 -500% <20% 15%

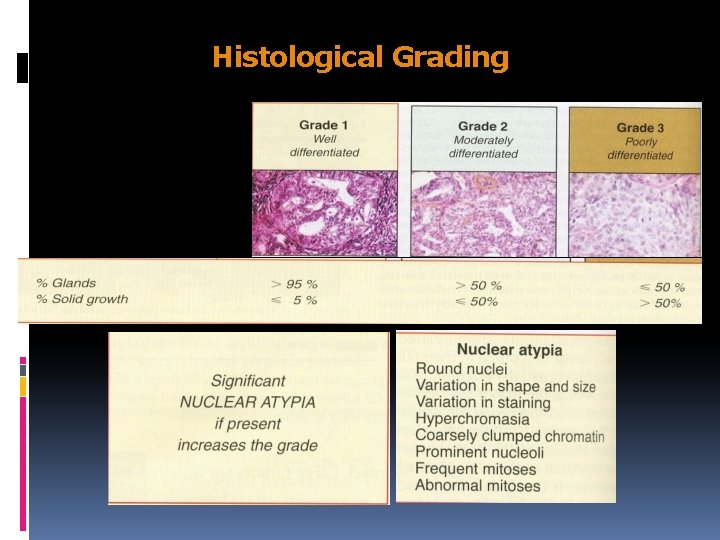
Endometrioid Carcinoma GRADING G 1. Well differentiated adenocarcinoma G 2. Differentiated adenocarcinoma with partly solid areas G 3. Predominantly solid or entirely undifferentiated carcinoma

Histological Grading

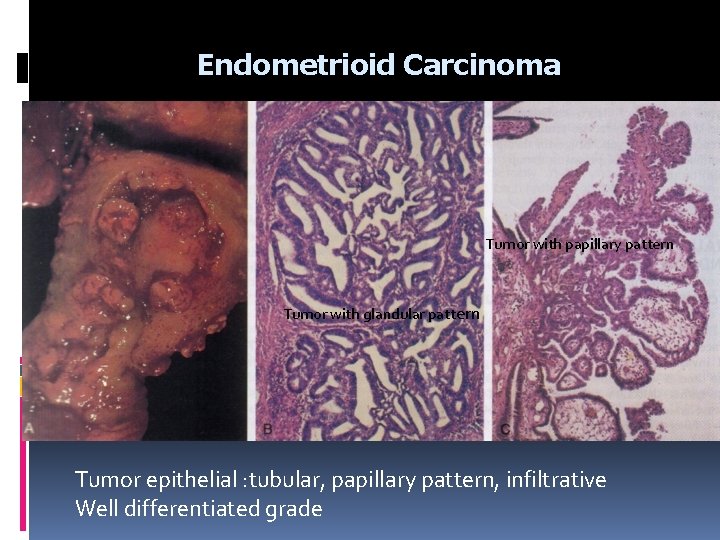
Endometrioid Carcinoma Tumor with papillary pattern Tumor with glandular pattern Tumor epithelial : tubular, papillary pattern, infiltrative Well differentiated grade

MYOMETRIAL TUMORS

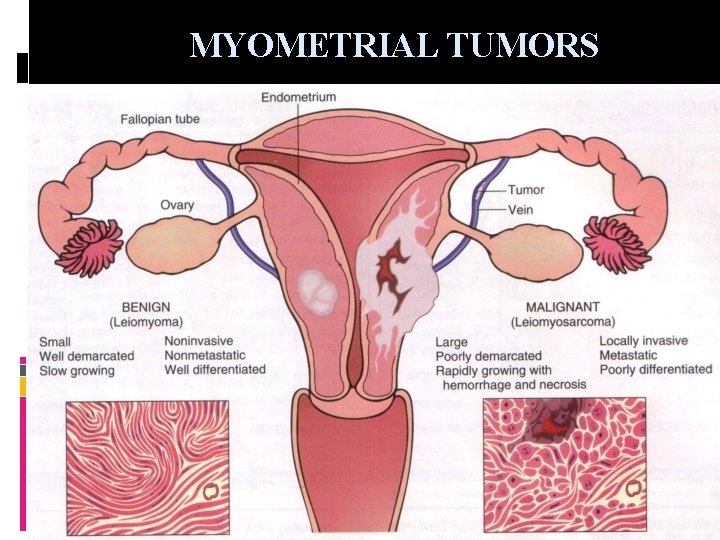
MYOMETRIAL TUMORS LEIOMYOMA Most common neoplasm in women during reproductive life (25%), 3 rd to 4 th decade, tending to decrease in size in menopause Malignancy is extremely unusual (<0. 1%) Symptom: pain of degeneration, bleeding, symptom related to size pressure on rectum and bladder, sensation of heaviness LEIOMYOSARCOMA Uncommon, as fleshy mass invading into uterine wall, or polypoid Arising de novo rather than from a pre-existing leiomyoma

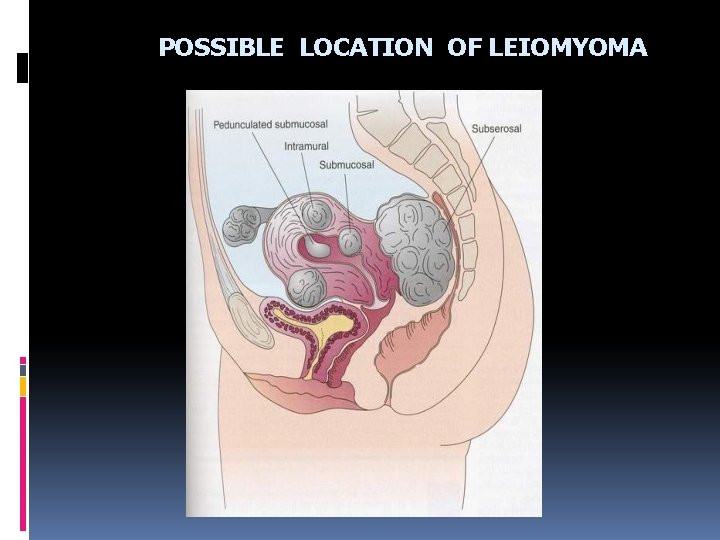
POSSIBLE LOCATION OF LEIOMYOMA

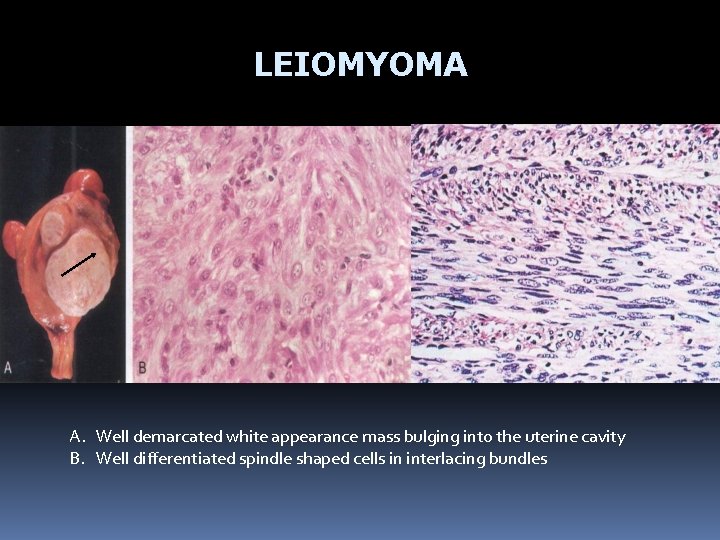
LEIOMYOMA A. Well demarcated white appearance mass bulging into the uterine cavity B. Well differentiated spindle shaped cells in interlacing bundles

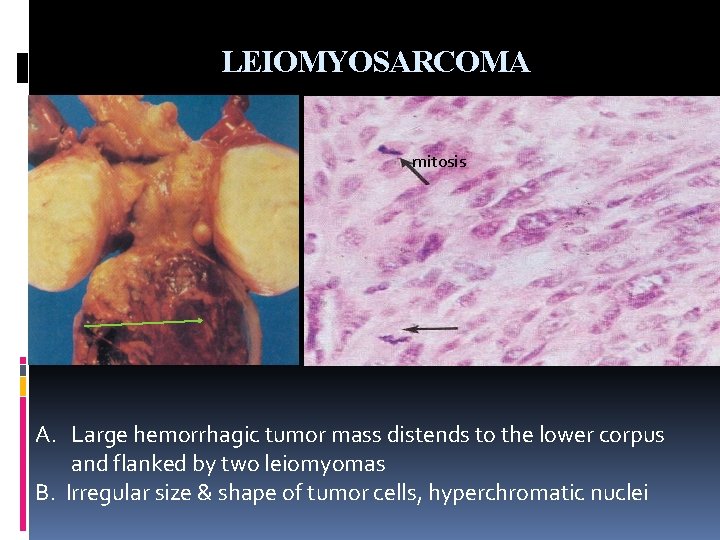
LEIOMYOSARCOMA mitosis A. Large hemorrhagic tumor mass distends to the lower corpus and flanked by two leiomyomas B. Irregular size & shape of tumor cells, hyperchromatic nuclei

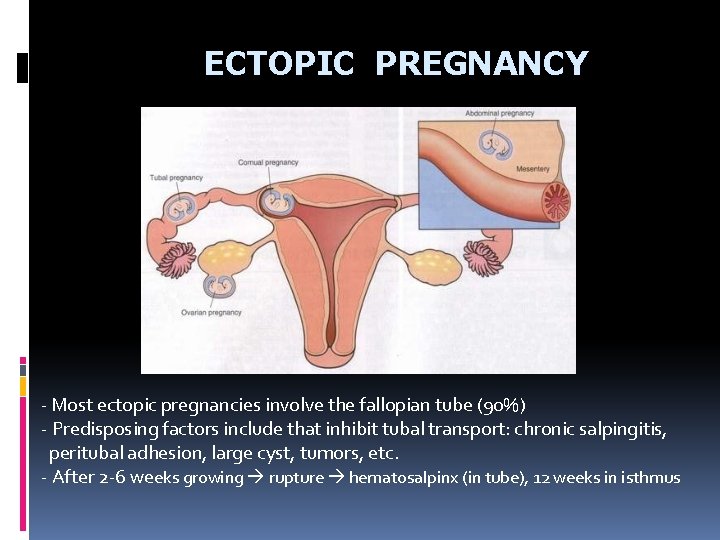
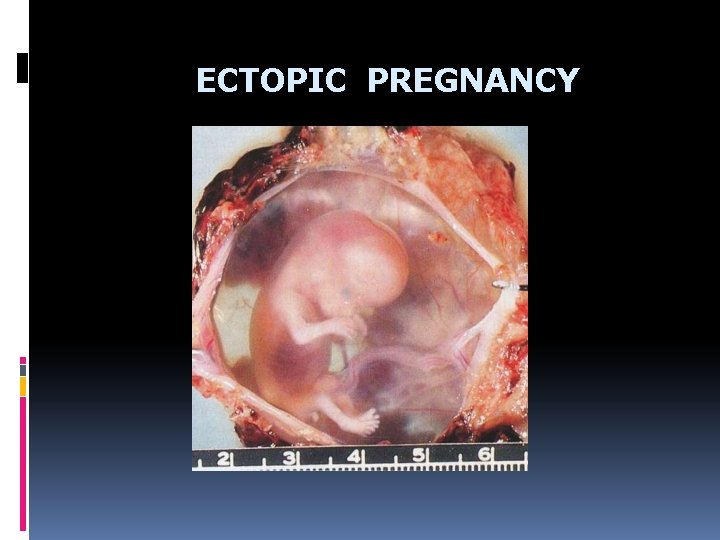
ECTOPIC PREGNANCY - Most ectopic pregnancies involve the fallopian tube (90%) - Predisposing factors include that inhibit tubal transport: chronic salpingitis, peritubal adhesion, large cyst, tumors, etc. - After 2 -6 weeks growing rupture hematosalpinx (in tube), 12 weeks in isthmus

ECTOPIC PREGNANCY

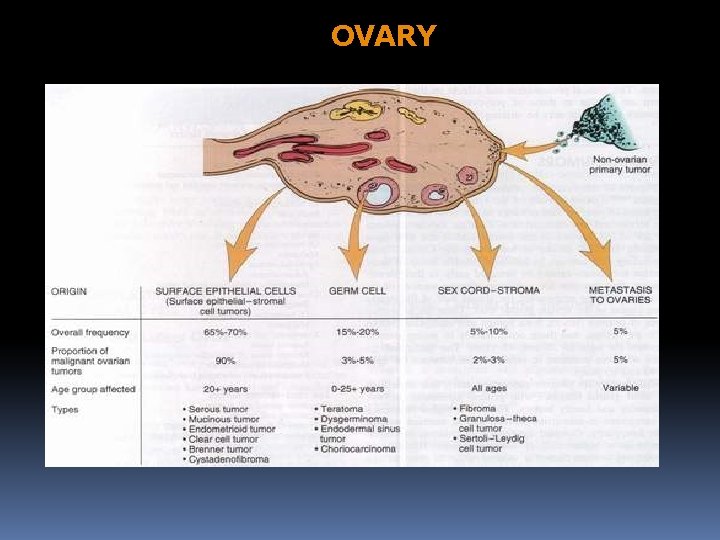
OVARY NON-NEOPLASTIC (80% are benign)

NON-NEOPLASTIC OVARIAN ENLARGMENT A. “Germinal” Inclusion Cyst - common cyst in pre-menopausal period, result of down growth and entrapment of the surface epithelium into the ovarian cortex B. Physiologic or Functional Cyst - follicle cyst - corpus luteum cyst - theca lutein cyst C. Polycystic ovaries D. Stromal Hyperplasia stromal hyperthecosis

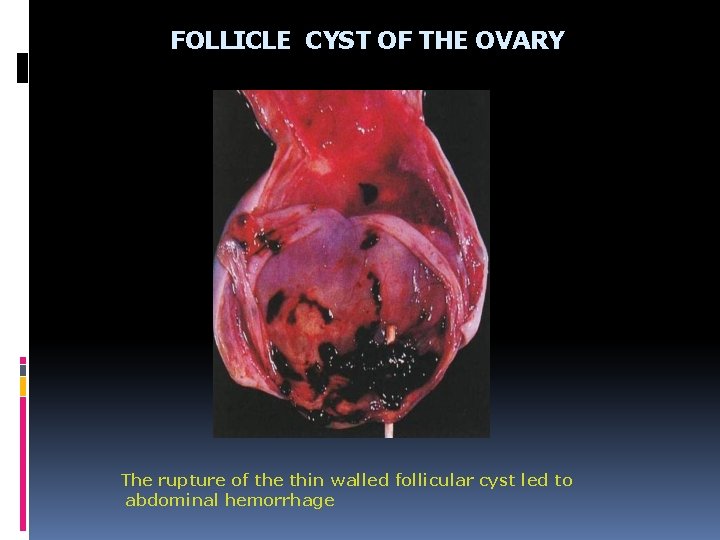
FOLLICLE CYST OF THE OVARY The rupture of the thin walled follicular cyst led to abdominal hemorrhage

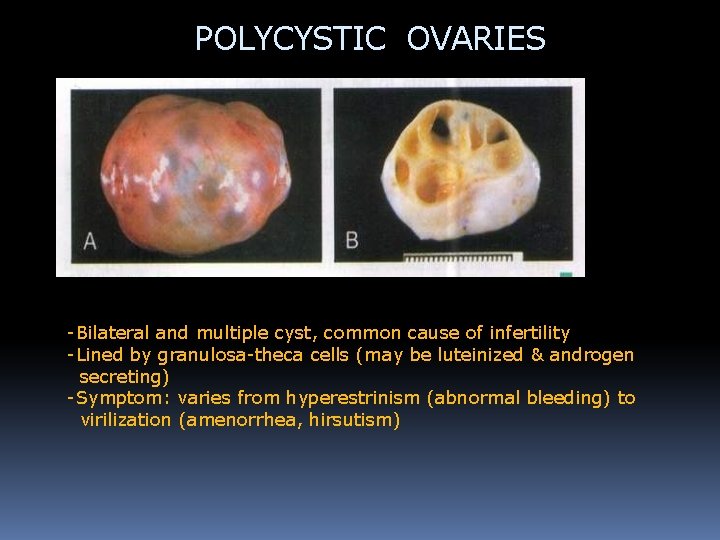
POLYCYSTIC OVARIES -Bilateral and multiple cyst, common cause of infertility -Lined by granulosa-theca cells (may be luteinized & androgen secreting) -Symptom: varies from hyperestrinism (abnormal bleeding) to virilization (amenorrhea, hirsutism)

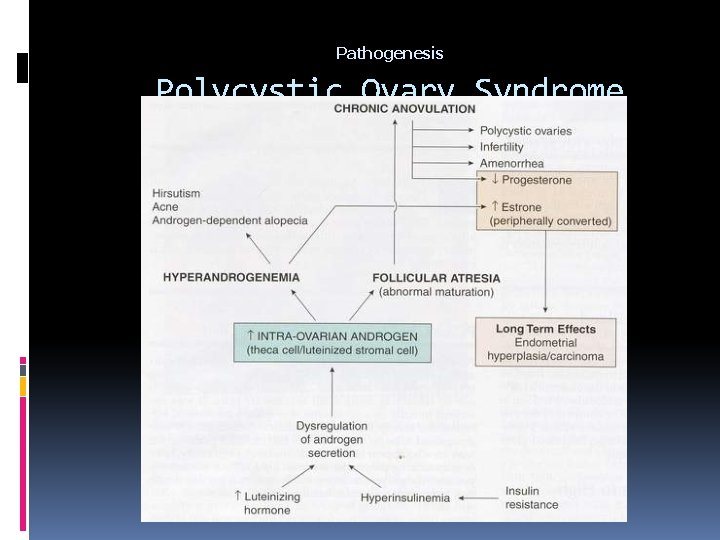
Pathogenesis Polycystic Ovary Syndrome

NEOPLASTIC OVARIAN ENLARGEMENT A. TUMORS DERIVED FROM SURFACE (GERMINAL) EPITHELIUM 1. Serous Tumors a. Serous cystadenoma b. Serous cystadenocarcinoma c. Serous borderline tumor 2. Mucinous Tumors a. Mucinous cystadenoma b. Mucinous cystadenocarcinoma c. Mucinous borderline tumor 3. Endometrioid Tumors 4. Brenner Tumors 5. Serous surface papilloma, cystadenofibroma, etc.

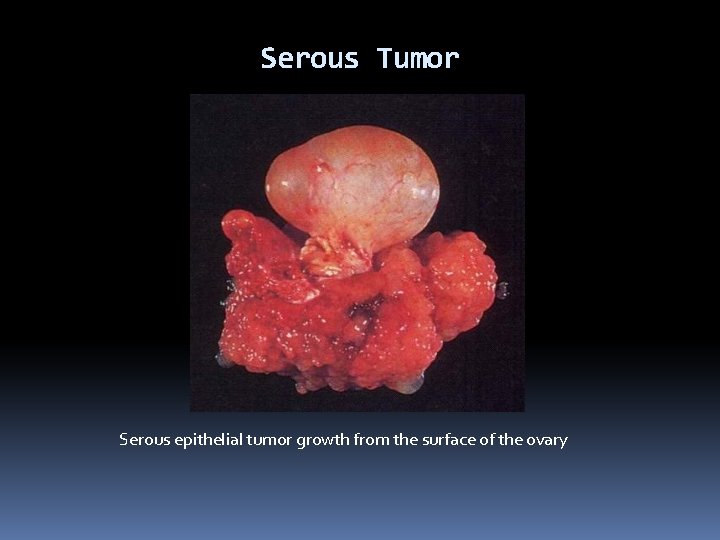
Serous Tumor Serous epithelial tumor growth from the surface of the ovary

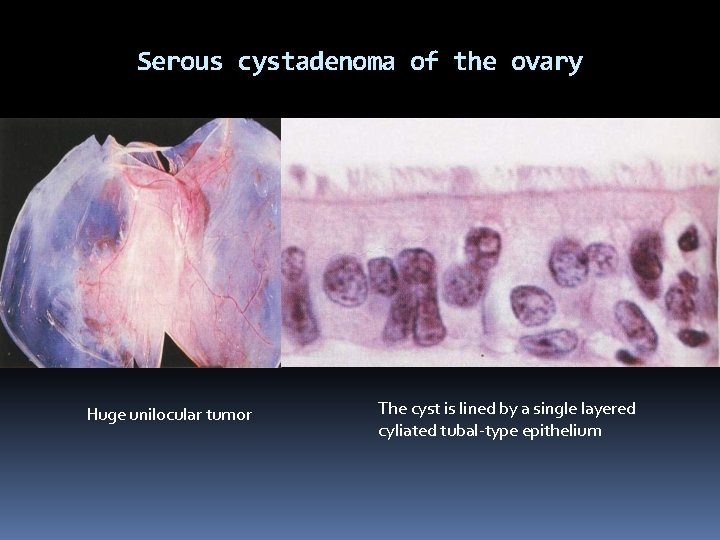
Serous cystadenoma of the ovary Huge unilocular tumor The cyst is lined by a single layered cyliated tubal-type epithelium

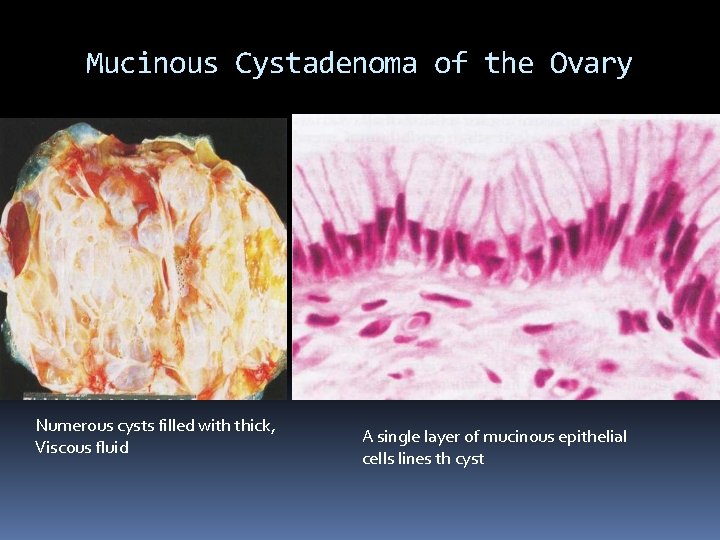
Mucinous Cystadenoma of the Ovary Numerous cysts filled with thick, Viscous fluid A single layer of mucinous epithelial cells lines th cyst

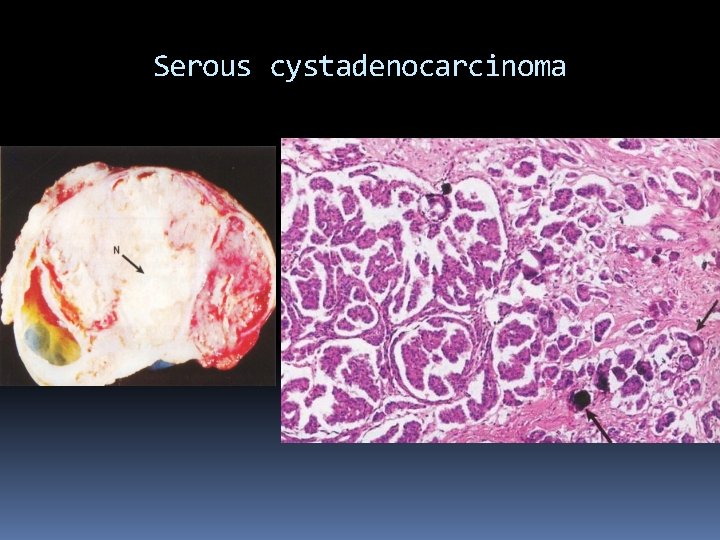
Serous cystadenocarcinoma

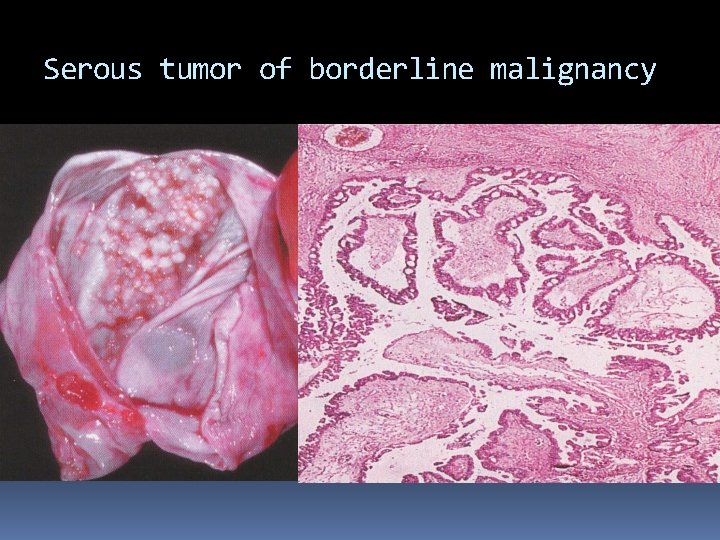
Serous tumor of borderline malignancy

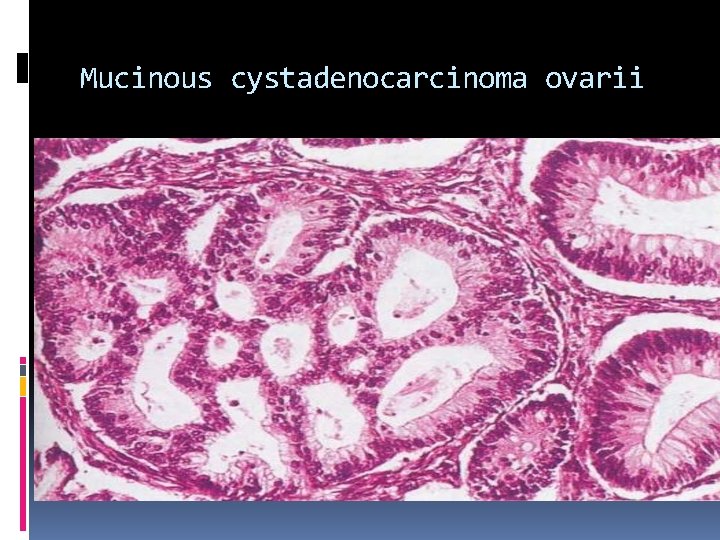
Mucinous cystadenocarcinoma ovarii

NEOPLASTIC OVARIAN ENLARGEMENT B. TUMORS DERIVED FROM SEX CORD/STROMA 1. Granulosa-Theca Cell Tumors 2. Fibroma 3. Sertoli-Leydig Cell Tumor 4. Hilus (hilar) Cell Tumor 5. Sertoli Cell Tumors

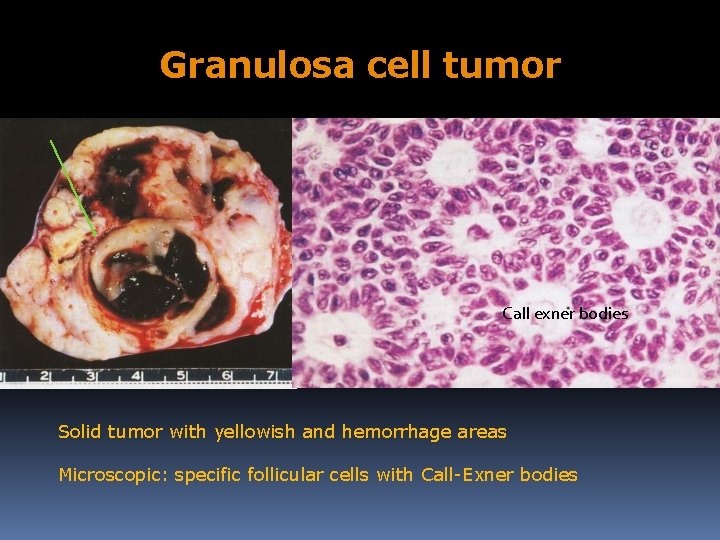
Granulosa cell tumor Call exner bodies Solid tumor with yellowish and hemorrhage areas Microscopic: specific follicular cells with Call-Exner bodies

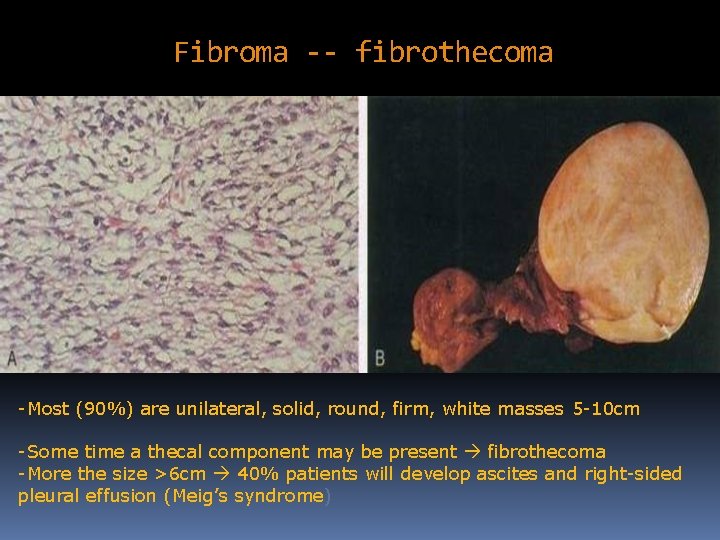
Fibroma -- fibrothecoma -Most (90%) are unilateral, solid, round, firm, white masses 5 -10 cm -Some time a thecal component may be present fibrothecoma -More the size >6 cm 40% patients will develop ascites and right-sided pleural effusion (Meig’s syndrome)

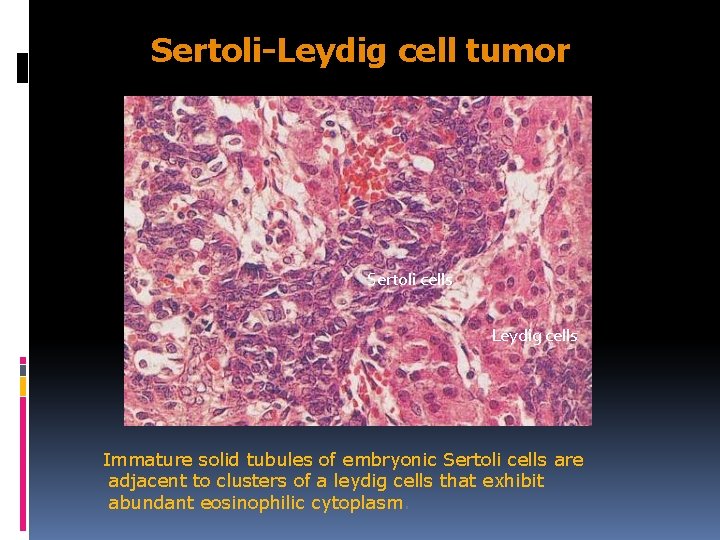
Sertoli-Leydig cell tumor Sertoli cells Leydig cells Immature solid tubules of embryonic Sertoli cells are adjacent to clusters of a leydig cells that exhibit abundant eosinophilic cytoplasm.

NEOPLASTIC OVARIAN ENLARGEMENT C. TUMORS DERIVED FROM GERM CELLS 1. Teratoma - Mature Cystic Teratoma - Immature (Malignant) Teratoma 2. Dysgerminoma 3. Endodermal Sinus (Yolk sac) Tumors 4. Embryonal Carcinoma 5. Choriocarcinoma D. METASTATIC TUMORS

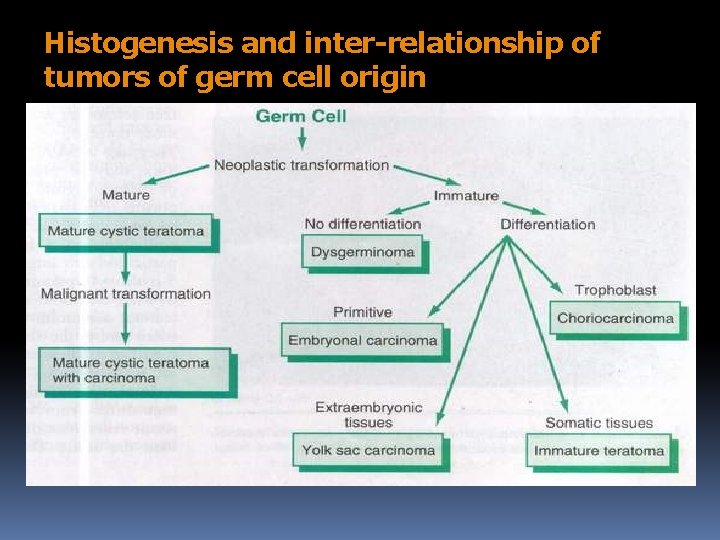
Histogenesis and inter-relationship of tumors of germ cell origin

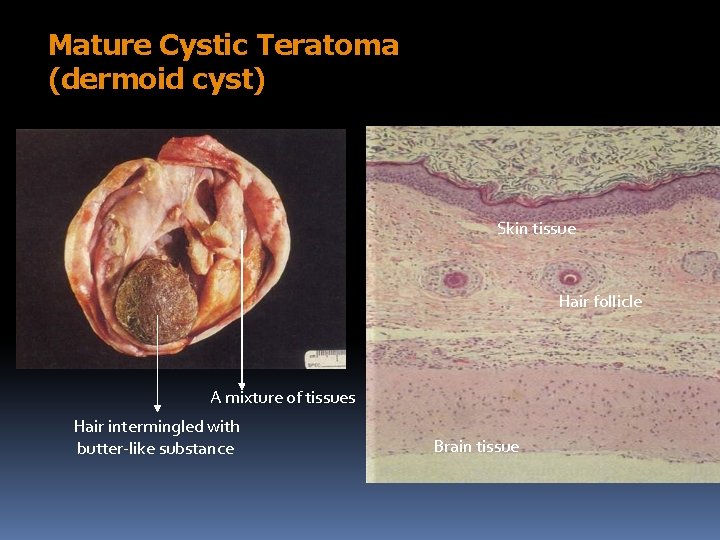
Mature Cystic Teratoma (dermoid cyst) Skin tissue Hair follicle A mixture of tissues Hair intermingled with butter-like substance Brain tissue

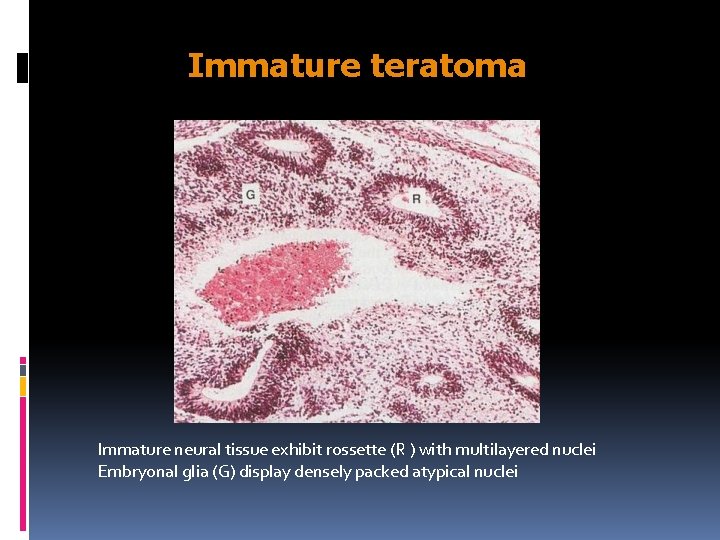
Immature teratoma Immature neural tissue exhibit rossette (R ) with multilayered nuclei Embryonal glia (G) display densely packed atypical nuclei

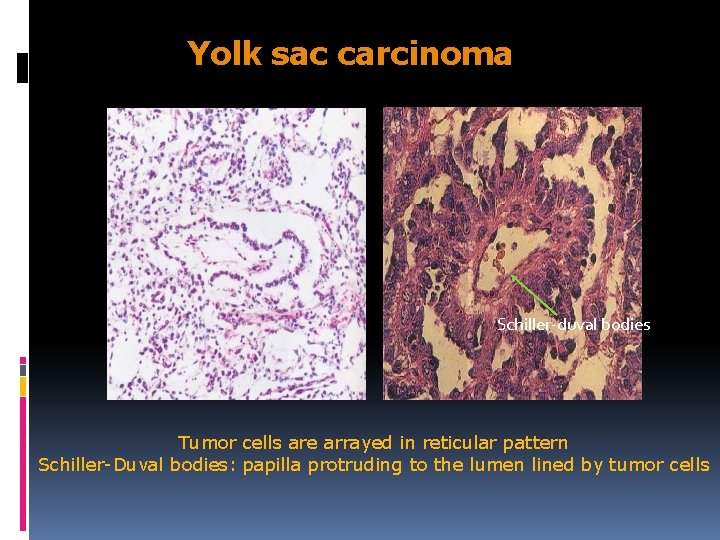
Yolk sac carcinoma Schiller-duval bodies Tumor cells are arrayed in reticular pattern Schiller-Duval bodies: papilla protruding to the lumen lined by tumor cells

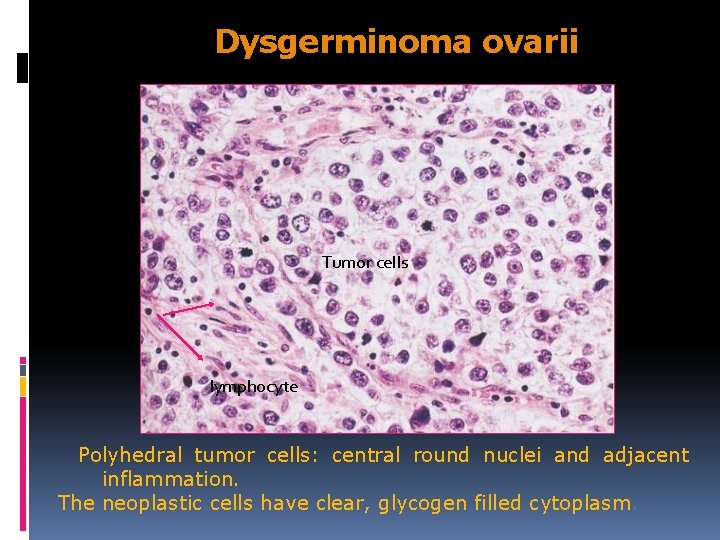
Dysgerminoma ovarii Tumor cells lymphocyte Polyhedral tumor cells: central round nuclei and adjacent inflammation. The neoplastic cells have clear, glycogen filled cytoplasm.

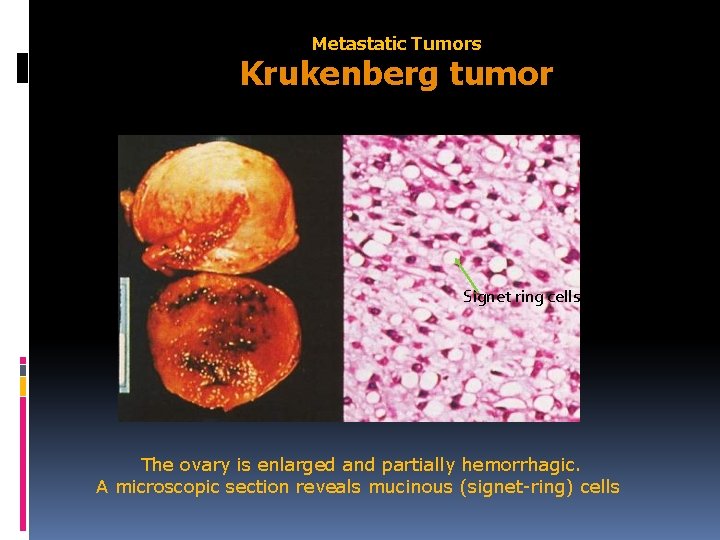
Metastatic Tumors Krukenberg tumor Signet ring cells The ovary is enlarged and partially hemorrhagic. A microscopic section reveals mucinous (signet-ring) cells

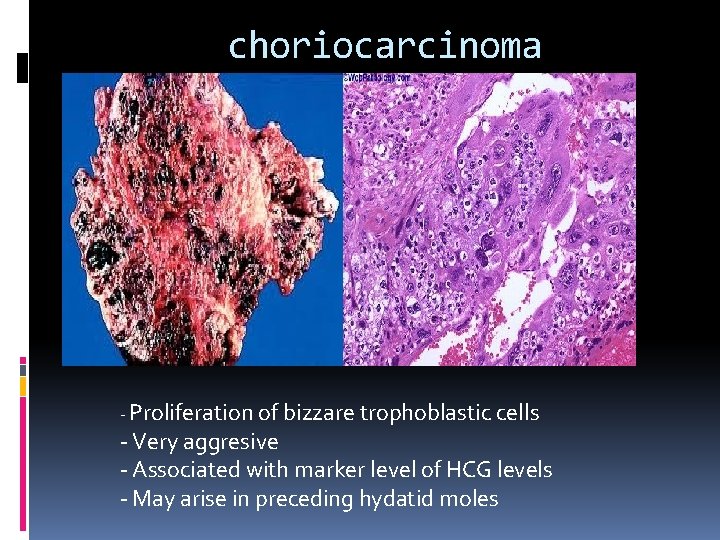
choriocarcinoma - Proliferation of bizzare trophoblastic cells - Very aggresive - Associated with marker level of HCG levels - May arise in preceding hydatid moles

OVARY