FEDIAF Guide to Good Practice for the Manufacture










- Slides: 11

FEDIAF Guide to Good Practice for the Manufacture of Safe Pet Food TRAINING PACKAGE Module II Quality Management System

2. QUALITY MANAGEMENT SYSTEM 2. 1 Quality and Pet food safety Policy and Objectives 2. 2 Pet Food Safety and Quality Management Manual 2. 3 Organisational Structure, Responsibility and Management Authority 2. 4 Management review 2. 5 Quality and Pet Food Safety Procedures 2. 6 Documentation Control 2. 7 Quality and Pet Food Safety Records

2. QUALITY MANAGEMENT SYSTEM (cont’d) 2. 8 Specifications and customer requirements/contract review 2. 9 Customer satisfaction 2. 10 Internal Audit and other verification of quality and pet food safety system 2. 11 Corrective/preventive actions 2. 12 Complaint handling 2. 13 Continuous improvement 2. 14 Internal Communication 2. 15 External Communication

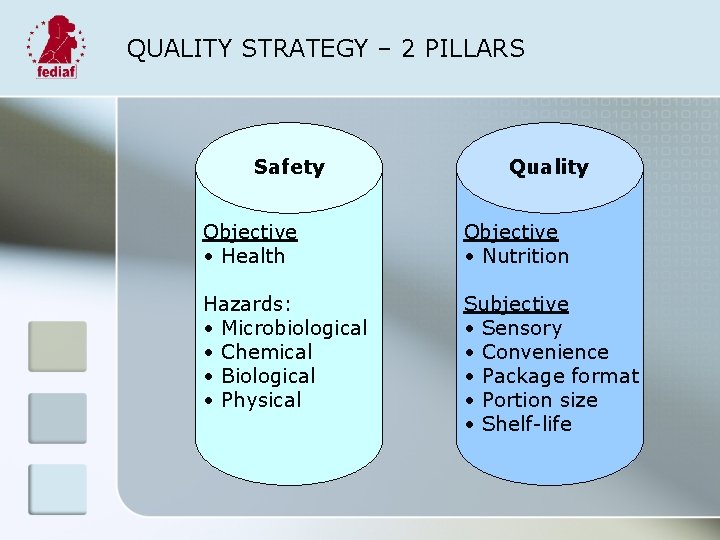
QUALITY STRATEGY – 2 PILLARS Safety Quality Objective • Health Objective • Nutrition Hazards: • Microbiological • Chemical • Biological • Physical Subjective • Sensory • Convenience • Package format • Portion size • Shelf-life

2. 1 Quality and Pet food safety Policy and Objectives What is the major objective? To assure that, when eaten according to its intended use, the pet food will not harm the animal Clearly defined and documented: • Produce safe and legal products • Responsibility towards its customers • Continuous review, improvement and communication • Senior management commitment

2. 2 Pet Food Safety and Quality Management Manual 2. 3 Organisational Structure, Responsibility and Management Authority 2. 2 Working methods in line with FEDIAF Code 2. 3 Structure clearly documented • Job description and responsibility chart • Directors provide adequate resources • How to monitor operations/trigger corrective actions • Information system of relevant issues

2. 4 2. 5 2. 6 Management review Quality and Pet Food Safety Procedures Documentation Control 2. 4 Quality management system regularly reviewed and improved 2. 5 Operate in accordance with written procedures • Documents legible, unambiguous and accessible 2. 6 Documents, records and data in place & controlled, such as • OPRPs and CCPs • Commercial documents/health certificates

2. 7 Quality and Pet Food Safety Records 2. 8 Specifications and customer requirements/contract review 2. 7 Demonstrate control of product safety/quality • Checks on OPRPs and CCPs, sampling procedures, analyses • Review, maintenance, storage of all records • Samples kept during normal consumption time 2. 8 Adequate specifications for : raw materials; packaging; processing; finished products; transport; etc.

2. 9 Customer satisfaction 2. 10 Internal Audit and other verification of quality and pet food safety system 2. 9 Use key performance indicators to monitor customer satisfaction 2. 10 Audit critical systems and procedures • Carry out internal audits and circulate results • Implement corrective actions

2. 11 Corrective / preventive actions 2. 12 Complaint handling 2. 11 Assess the cause of non-conformity • Problems used to re-engineer processes • Corrective actions documented and undertaken timely • Preventive measures based on HACCP • Production lines based on HACCP study 2. 12 Registration and management of complaints • System for prompt recall of products • Used to improve products, review HACCP and CCPs

QUALITY RESULTS How to measure quality results? Examples include: • Monitor pathogen agents and contaminants • Analyse trends of the quality parameters • Market research • Set a goal for quality complaints