Fecal Microbiota Transplantation FMT For Recurrent Clostridium difficile

Fecal Microbiota Transplantation (FMT) For Recurrent Clostridium difficile Infection Nedret Copur-Dahi, MD Associate Clinical Professor of Medicine UCSD, Section of Gastroenterology
Objectives v Summarize C. difficile infection v Understand when fecal transplantation is an appropriate treatment v Discuss the different delivery methods of FMT v Identify future treatment options

Clostridium difficile Brief Overview

Clostridium difficile v. Anaerobic, spore-forming, toxin-producing, gram + rod v. Transmission: via fecal-oral route v. Asymptomatic carriers; 5– 15% of healthy adults 84. 4% in newborns/infants 57% in residents in LTCF

Evolving Magnitude �Rising incidence; >450, 000 cases in 2011 CDC data Rising CDI-related death rate; 29, 000 in 2011 Annual health care costs; up to $5. 9 billion/year Lessa FC, et al. N Engl J Med. 2015; 372: 825 -834 �Has become the most common nosocomial pathogen; 12. 1% of all hospital acquired infections Magill, et al. N Engl J Med. 2014; 370(13): 1198 -1208
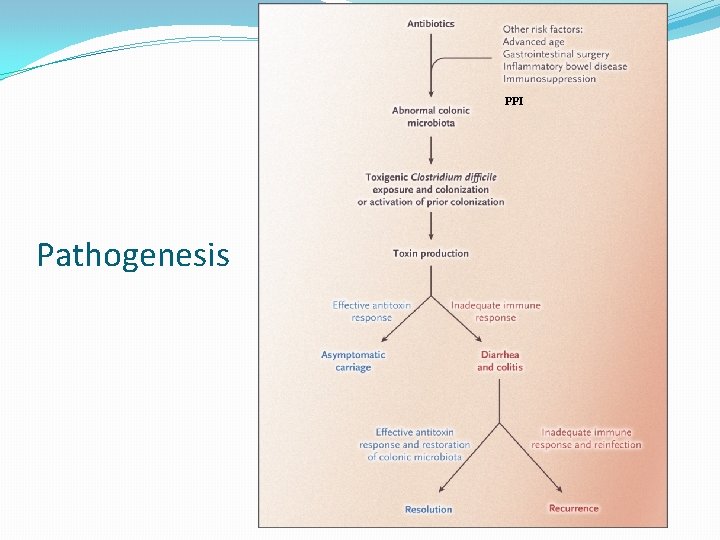
PPI Pathogenesis

Antibiotics to Precipitate Sleisenger and Fordtrans Gastrointestinal and liver disease, 9 th ed. Saunders: Philadelphia, 2010: 1889 -1903.
Recurrence �Caused by same or different strain from the 1 st infection and due to insufficient immune response and/or persistent dysbiosis �Clostridium difficile recurs in 20 - 25 % 1, 2 ; 40 -60% after 1 st recurrence � 10% of patients will not respond to standard therapy 1. Kelly et al. N Engl J Med 2008; 359: 1932 -1940. 2. Khanna et al. Am J Gastroenterol 2012; 107: 89 -95.
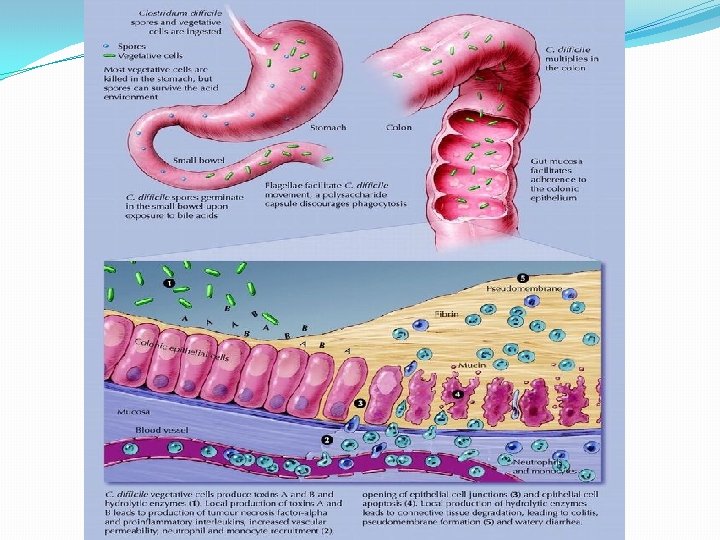
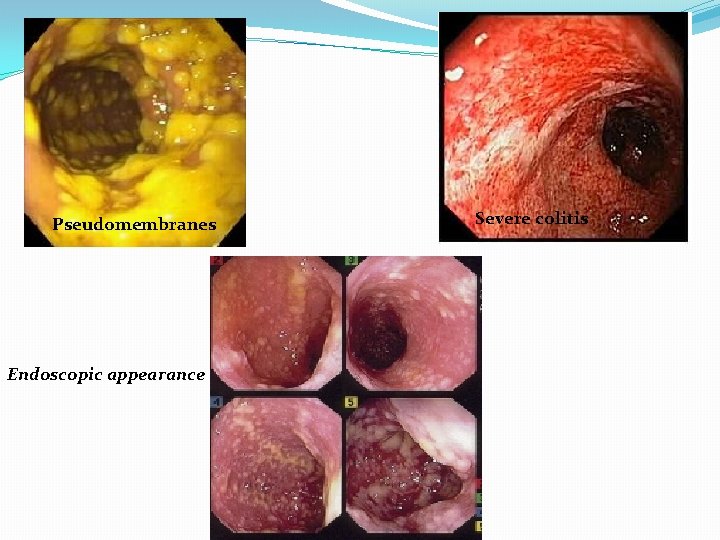
Pseudomembranes Endoscopic appearance Severe colitis
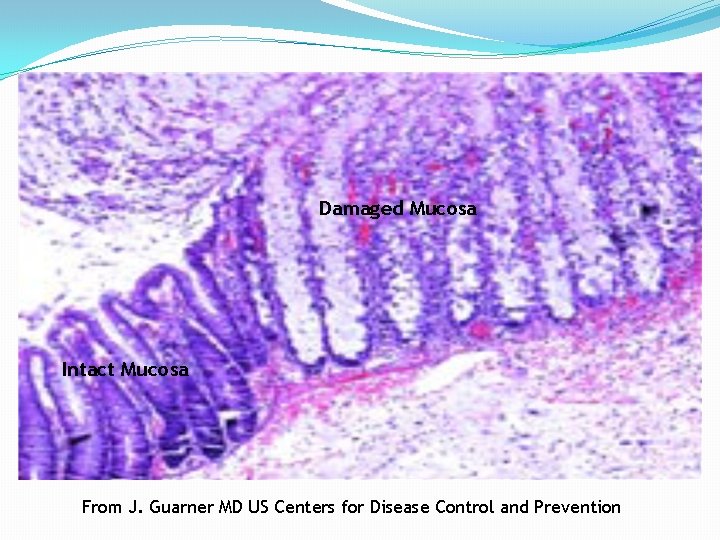
Damaged Mucosa Intact Mucosa From J. Guarner MD US Centers for Disease Control and Prevention
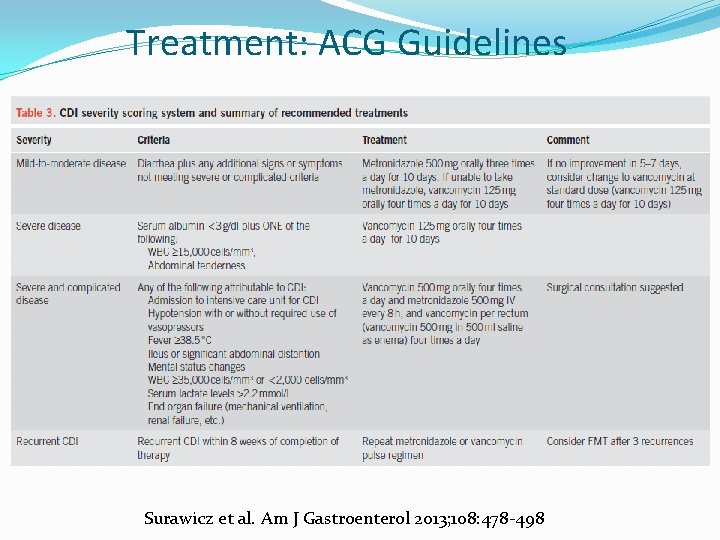
Treatment: ACG Guidelines Surawicz et al. Am J Gastroenterol 2013; 108: 478 -498
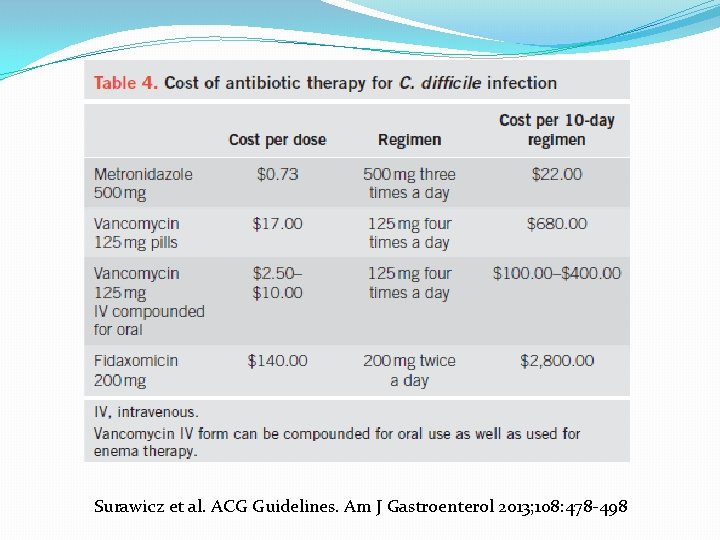
Surawicz et al. ACG Guidelines. Am J Gastroenterol 2013; 108: 478 -498
Gut Microbiota 1000 -1200 species, 1014 bacteria
Gut Microbiota �Most important first-line host defense against pathogen colonization resistance to bacterial colonization stimulation of the mucosal immune system �Infectious agents and antibiotic use disruption of the normal gut microbiota (dysbiosis) initial or recurrent C. difficile infection = deficient in Bacteroides and Firmicutes 1, 2 1. Nat Rev Microbiol. 2011 Jan; 9(1): 27 -38. Epub 2010 Nov 29 2. Chang JY, Antonopoulos DA, Kalra A, et al. J Infect Dis 2008; 197: 435.

Fecal Microbiota Transplantation *** the ultimate probiotic ***

FMT History � 4 th Century: Human fecal suspension for food poisoning or severe diarrhea � 16 th -17 th Century: yellow soup to treat diarrheal illnesses � Transfaunation by veterinarians � 1958 in Denver: fecal enema for fulminant, life-threatening pseudo-membranous enterocolitis � 1983: FMT enema in 65 yo woman with CDI � 1991: Via NG tube � 200 os: Colonoscopy
Is FMT Durable? �Infused donor fecal microbiota: stable in composition over a 24 -week period prolonged cure rate of 91 -94% immediate and complete resolution of symptoms Yoon SS, Brandt LJ. J Clin Gastroenterol 2010; 44: 562
Drekonja D, et al. FMT for CDI: A Systematic Review. Ann Intern Med May, 2015; 162: 630. Minneapolis VAMC.
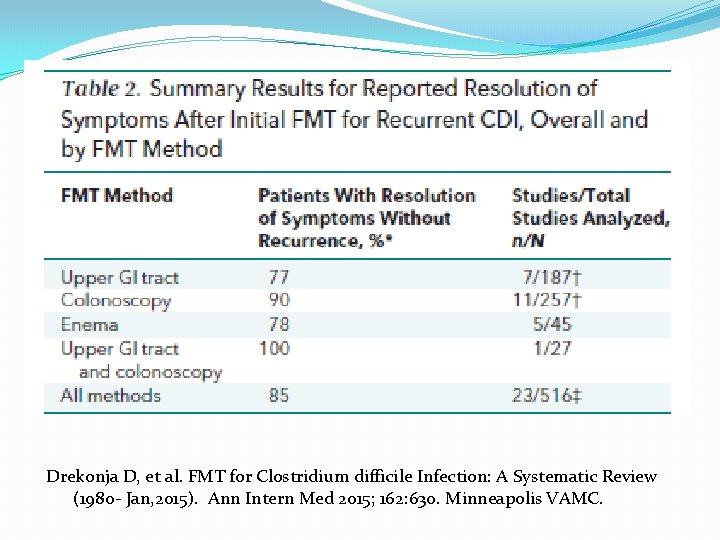
Drekonja D, et al. FMT for Clostridium difficile Infection: A Systematic Review (1980 - Jan, 2015). Ann Intern Med 2015; 162: 630. Minneapolis VAMC.

Patient Selection �Severe and recurrent C. difficile infection �Failed multiple (≥ 3) attempts at conventional antibiotic therapy (vancomycin, metronidazole, fidaxomicin , or 6 -8 week vancomycin taper, or vancomycin pulsed regimen of 10 -13 doses or vancomycin followed by rifaximin chaser for 2 weeks). �Refractory moderate to severe C. difficile diarrhea, failing vancomycin after >1 week

FMT Protocol – The Past

Protocol �No standardization in the preparation and administration of the fecal suspension. �Every facility prepared their own protocol.
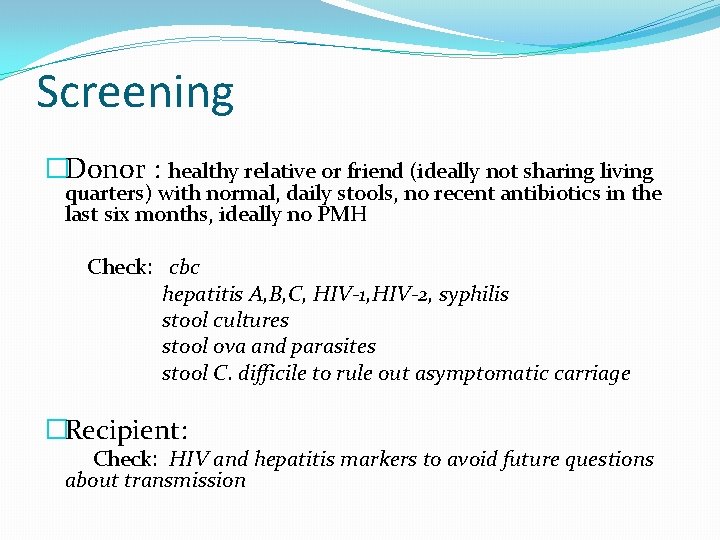
Screening �Donor : healthy relative or friend (ideally not sharing living quarters) with normal, daily stools, no recent antibiotics in the last six months, ideally no PMH Check: cbc hepatitis A, B, C, HIV-1, HIV-2, syphilis stool cultures stool ova and parasites stool C. difficile to rule out asymptomatic carriage �Recipient: Check: HIV and hepatitis markers to avoid future questions about transmission
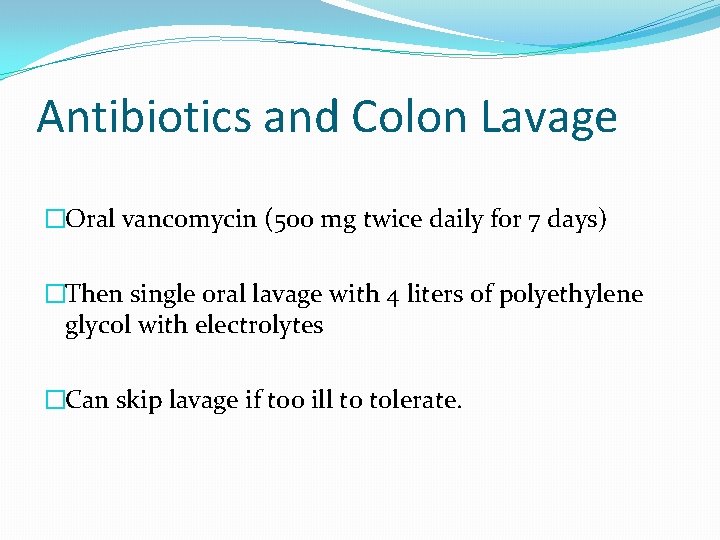
Antibiotics and Colon Lavage �Oral vancomycin (500 mg twice daily for 7 days) �Then single oral lavage with 4 liters of polyethylene glycol with electrolytes �Can skip lavage if too ill to tolerate.

Administration Methods Retention enema Colonoscopy Nasogastric/nasoduodenal tube Oral !!!

Administration via LGIT

Preparation and Methods 200 -300 g of donor stool suspended in 200 -300 m. L of normal saline homogenize briefly (2 min) in kitchen blender to a liquid consistency give via enema within 10 minutes or filter then infuse via colonoscopy into TI. retain for at least 6 hours (loperamide pretreatment) and then follow a high fiber diet repeat the process daily for five days (not practical) �In less severe cases, a single colonoscopic infusion to the colon is adequate 1. 1. Yoon SS, Brandt LJ. J Clin Gastroenterol 2010; 44: 562
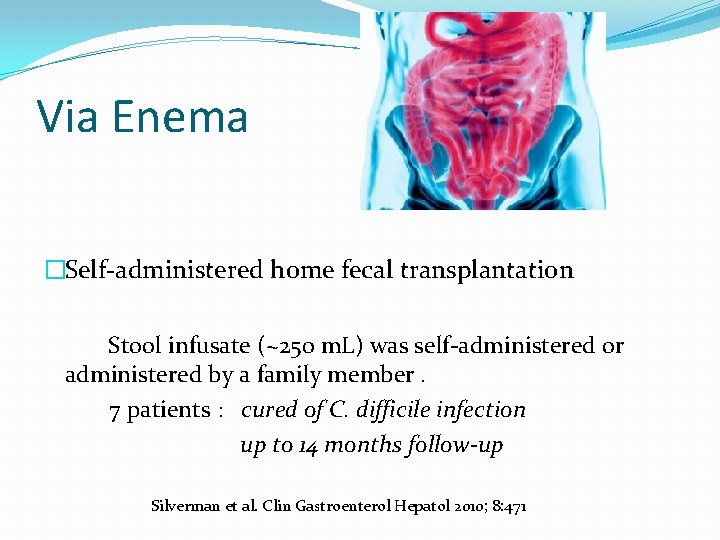
Via Enema �Self-administered home fecal transplantation Stool infusate (~250 m. L) was self-administered or administered by a family member. 7 patients : cured of C. difficile infection up to 14 months follow-up Silverman et al. Clin Gastroenterol Hepatol 2010; 8: 471

Probiotic Infusion Package for bowel -PIP 10 Starter pack for 10 home infusions.
Via Colonoscope � Greater area of recolonization greater capacity to inhibit spore formation proximal to the splenic flexure. � Deliver the microbiota to the distal small bowel where C. difficile can reside. � 26 patients; 92% symptom free average follow-up of 10. 7 months 1 Kelly et al. J Clin Gastroenterol. . 2012 Feb; 46(2): 145 -9 � RCT of 46 patients; received full course vanco then - donor stool (cure rate : 92%) or - autologous stool (63%) or - placebo Kelly et al. Ann Intern Med. 2016; 165(9): 609.
Adverse Reactions �Transient and mild �Post-enema symptoms: Abdominal gurgling, gas, and noise 1 �Colonoscopy complications 1. Bowden TA Jr, Mansberger AR Jr, Lykins LE. Am Surg 1981; 47: 178
Administration via UGIT

Preparation and Method �Patient and stool preparation is similar. �PPI (omeprazole 20 mg) evening before and morning of the instillation to decrease gastric acid �NG or NJ tube is placed. �The positioning is confirmed by x-ray and Gastrografin follow-through. �A single instillation of 25 to 30 g of stool diluted in 50 m. L of saline.
Via Nasogastric or Nasoduodenal Tube �Can deliver the bacteria to the distal small bowel and throughout the colon. � 43 patients; vancomycin 500 mg x 4/day for 4 days, bowel lavage and duodenal infusion (81% resolution without relapse at 10 weeks) vancomycin 500 mg x 4/day for 14 days (31% resolution) vancomycin 500 mg x 4/day for 14 days with bowel lavage (23% resolution) van Nood et al. N Engl J Med. 2013; 368(5): 407.

Adverse Reactions �Aspiration of the gastric contents !!!!!

Via Oral � A fecal suspension in normal saline using a commercial blender. � Concentrated by centrifugation and resuspended in saline at onetenth the volume. � Pipetted into size 0 capsules (650 µL), closed and then sealed in size 00 capsules. � Acid resistant capsules were stored frozen at − 80°C (− 112°F). 1 -2 hours prior to administration, they were transferred to − 20°C, then transported on dry ice. � 30 capsules contained sieved, concentrated material derived from a mean of 48 g of fecal matter. Youngster et al. JAMA. 2014; 312(17): 1772 -1778. Division of Infectious Diseases, Massachusetts General Hospital.

Via Oral � 20 patients �Total 30 frozen FMT capsules (2 consecutive days). �Diarrhea resolved in 14 of 20 patients with single treatment and 4 of 6 nonresponders who were retreated. �No serious adverse events within six months followup. �Larger studies are needed and in process. Youngster et al. JAMA 2014

FMT Appears to Be Safe �No complications reported in published series �Risks of aspiration with nasogastric tube �Colonoscopy procedural risks �Potential transmission of infectious agents contained in the stool. Case reports of norovirus GE, E. coli and polymicrobial bacteremia, flare of quiescent UC, herpes zoster after FMT. � 77 patient with colonoscopic FMT: 3 mo to >10 years follow-up 4 autoimmune diseases (RA, ITP, Sjogren’s, peripheral neuropathy). No clear relationship. Brandt et al. Am J Gastroenterol 2012; 107: 1079 -87

Post-FMT Recurrence �No recurrence in 1 to 3 years in most patients even though a number of patients have subsequently required antibiotics for unrelated infections. Borody TJ, Warren EF, Leis SM, et al. J Clin Gastroenterol 2004; 38: 475

The Present
The Present - Open. Biome �Non-profit organization established in 2012 in Medford, MA; currently supported largely by charitable donations. �Increased the accessibility and ease of FMT �Donors: young researchers and scientists within the MIT, Harvard, and Tufts communities, and young professionals from the Tufts University area. Screened and tested every 60 days. �Three products: FMP 250 : 250 m. L fecal microbiota preparation for lower delivery (colonoscopy, enema). FMP 30 : 30 m. L fecal microbiota preparation for upper delivery. FMT Capsule G 3 (30 frozen capsules consumed within 90 min)
The Future

Live Biotherapeutic Microbiota Preparations
Refined FMT: Synthetic Preparations �Human synthetic stool mixture: 33 different bacteria two elderly patients symptom free for 6 months Petrof et al. Microbiome. 2013; 1(1): 3. �Ongoing proof-of-concept study, estimated enrolment of 30 and completion in mid-2017, results pending NCT 01372943

Refined FMT: Fecal Spore Preparations �Encapsulated spore fractions containing 50 species from the Firmicutes bacteria phylum were prepared from donor stool for oral delivery as SER-109. � 30 patients treated with 2 doses of encapsulated spores Absence of recurrence in 8 weeks follow-up was 87% Khanna S, et al. Aliment Pharmacol Ther. 2016; 44: 715 -727. � Interim data from phase 2 placebo-controlled ECOSPOR study of 89 patients failed to support a benefit after 8 -weeks. Seres Therapeutics; NCT 02437487 � Ongoing expanded access study, ECOSPOR 2 is enrolling the patients who had recurrence. NCT 02437500
Refined FMT: Fecal Spore Preparations �The SER-262: first synthetically-derived and designed microbiome therapeutic ever to reach clinical-stage development Phase 1 b study 24 -week randomized, placebo-controlled, dose escalation study expected to enroll approximately 60 patients who have experienced a first episode of CDI. The primary endpoint of the study will compare the CDI recurrence rate between the SER-262 and placebo groups at up to 8 weeks after dosing. SER-262 (SER-262 -001 STUDY)

Nontoxigenic C. difficile Spores �Multi-center phase 2 RCT of nontoxicogenic C. difficile (NTCD) M 3 spores in an oral liquid. 173 patients with primary CDI or first recurrence. Treated with metronidazole, vanco or both, then randomized into 4 groups (3 M 3 dosing regimens and placebo). 5% recurrence of 43 patients receiving 10 7 spores/d for 7 days vs 30% of 43 placebo patients. 31% recurrence when not colonized (similar to placebo). Gerding et al. JAMA. 2015; 313(17): 1719 -1727
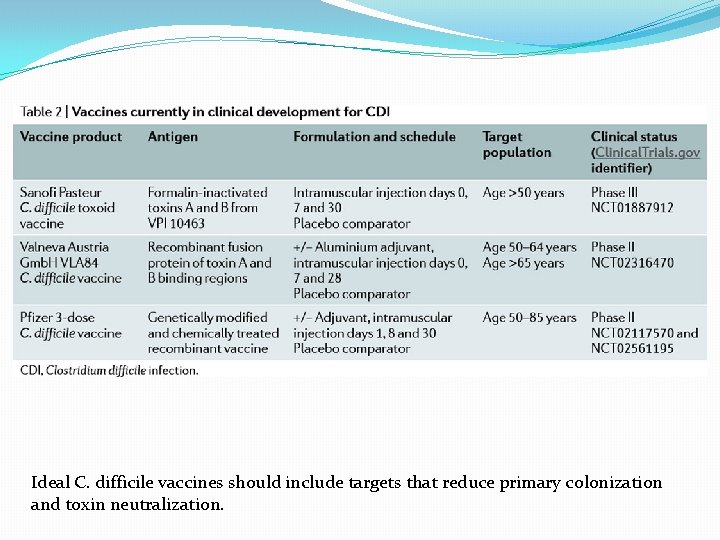
Ideal C. difficile vaccines should include targets that reduce primary colonization and toxin neutralization.

Summary �Important to restore the normal fecal microbiota to resolve refractory C. difficile infection. �Fecal microbiota transplantation durably restores the normal microbiota. �FMT should be considered in patients with 3 rd recurrence after a pulsed vancomycin regimen. �FMT appears to be safe. �Risk of transmission of infectious agents may be reduced by obtaining stool from healthy donors and by testing stool and blood for common viral, bacterial pathogens and parasites.

Future Research �FMT treatment of IBD and IBS; promising results from case series. (randomized controlled trials are needed to validate clinical observations) �Is FMT a valid treatment for a variety of non-GI diseases like Parkinson’s, autism, Multiple Sclerosis, chronic fatigue, and obesity among others?

Future Research Open. Biome Active Studies ENROLLING • Longitudinal Safety Study of FMT (STOOL Study) • CDI in Liver Transplant Recipients • recurrent CDI • Adult Active Moderate Ulcerative Colitis • Diarrheal-Predominant Irritable Bowel Syndrome 1 • Diarrheal. Predominant Irritable Bowel Syndrome 2 • HIV-Associated Diarrhea • Obesity • Pediatric Ulcerative Colitis • Post-Operative Crohn’s Disease • Pouchitis • Primary Sclerosing Cholangitis COMPLETED ENROLLMENT • Adult Ulcerative Colitis • Hepatic Encephalopathy • Inflammatory Bowel Disease (Ulcerative Colitis and Crohn's Disease) Planning and Design CDI • Geriatric CDI Prophylaxis • Primary CDI • Severe-Complicated CDI Non-CDI • Allergies • Autoimmune Disease • Antibiotic Resistant Bacteria • Antibiotic Dependent Pouchitis • Gut. Brain Conditions • Infectious Disease • Metabolic Disease • Pediatric Crohn's Disease • Pouchitis Diagnostics and Biomarkers CDI • CDI Detection Non-CDI • Autism • IBD Biomarkers • Nutrition • Parkinson’s Disease • Rotavirus Assay Development • Sleep Deprivation “A poop capsule a day keeps the doctor away ? ? ? ”
Every part of our body interacts with every other part. Our colony of micro-organisms is no exception.

Questions?
- Slides: 54