FDA Regulatory Requirements and FDAs Bioresearch Monitoring BIMO

FDA Regulatory Requirements and FDA’s Bioresearch Monitoring (BIMO) Program Constance Lewin, M. D. , M. P. H. Associate Director for Human Subject Protection Acting Branch Chief, Good Clinical Practice Branch 1 Division of Scientific Investigations Office of Medical Policy Center for Drug Evaluation and Research University of Miami Statewide Conference Miami, Florida April 21, 2006
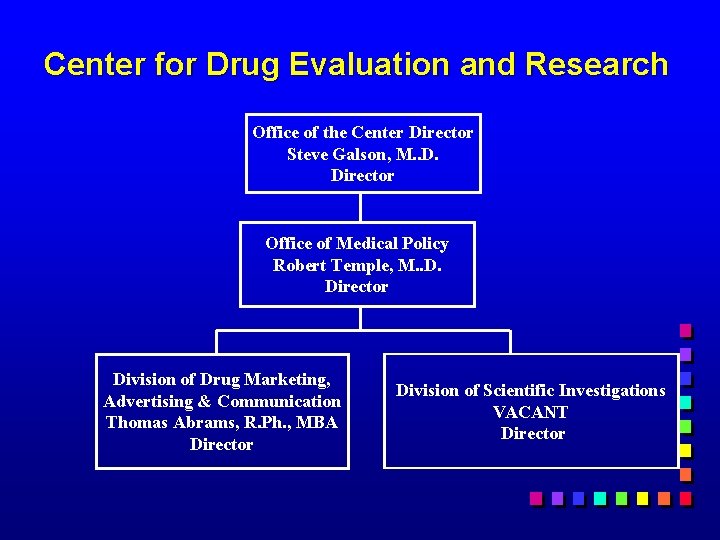
Center for Drug Evaluation and Research Office of the Center Director Steve Galson, M. . D. Director Office of Medical Policy Robert Temple, M. . D. Director Division of Drug Marketing, Advertising & Communication Thomas Abrams, R. Ph. , MBA Director Division of Scientific Investigations VACANT Director
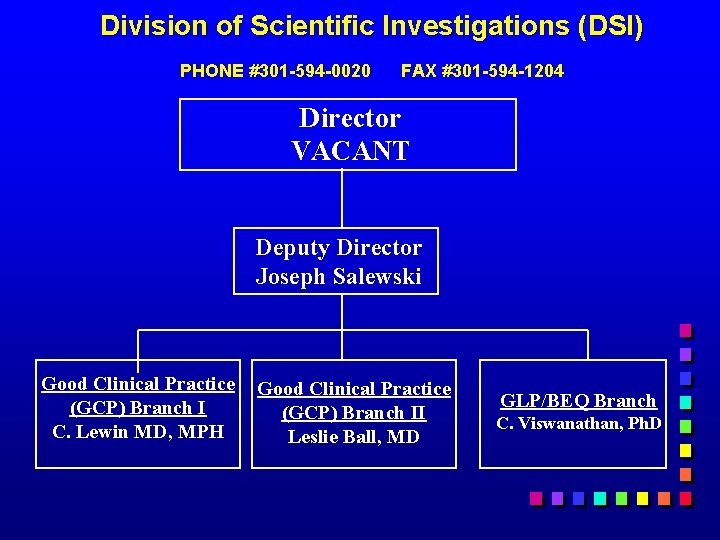
Division of Scientific Investigations (DSI) PHONE #301 -594 -0020 FAX #301 -594 -1204 Director VACANT Deputy Director Joseph Salewski Good Clinical Practice (GCP) Branch I C. Lewin MD, MPH Good Clinical Practice (GCP) Branch II Leslie Ball, MD GLP/BEQ Branch C. Viswanathan, Ph. D

Agenda FDA regulatory requirements for clinical investigators (CIs), sponsors and institutional review boards (IRBs) involved in FDA-regulated clinical research n Overview of FDA’s Bioresearch Monitoring Program n

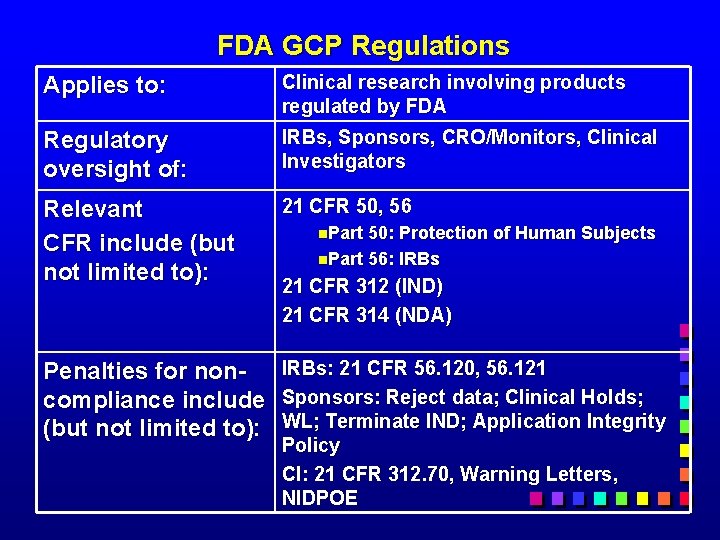
FDA GCP Regulations Applies to: Clinical research involving products regulated by FDA Regulatory oversight of: IRBs, Sponsors, CRO/Monitors, Clinical Investigators Relevant CFR include (but not limited to): 21 CFR 50, 56 Penalties for noncompliance include (but not limited to): IRBs: 21 CFR 56. 120, 56. 121 Sponsors: Reject data; Clinical Holds; WL; Terminate IND; Application Integrity Policy CI: 21 CFR 312. 70, Warning Letters, NIDPOE n. Part 50: Protection of Human Subjects n. Part 56: IRBs 21 CFR 312 (IND) 21 CFR 314 (NDA)
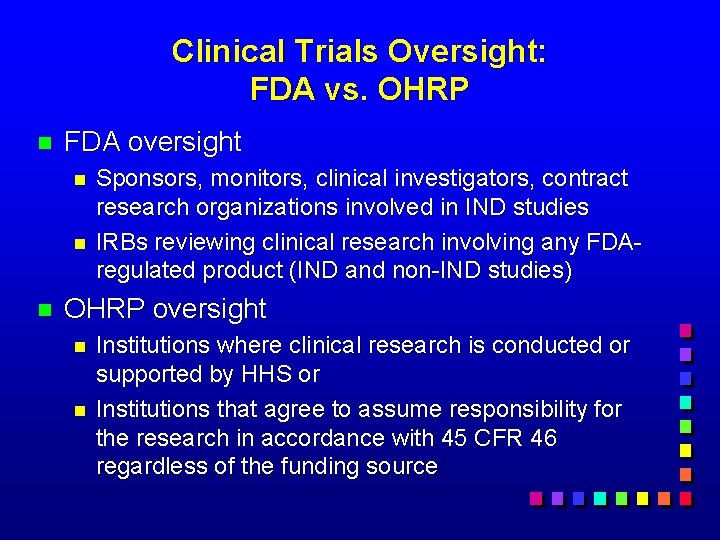
Clinical Trials Oversight: FDA vs. OHRP n FDA oversight n n n Sponsors, monitors, clinical investigators, contract research organizations involved in IND studies IRBs reviewing clinical research involving any FDAregulated product (IND and non-IND studies) OHRP oversight n n Institutions where clinical research is conducted or supported by HHS or Institutions that agree to assume responsibility for the research in accordance with 45 CFR 46 regardless of the funding source

When Do I Need an IND? CFR 312. 2 (b) Exemptions The clinical investigation of a drug product that is lawfully marketed in the United States is exempt from the requirements of this part if all of the following apply: (i) The investigation is not intended to be reported to FDA as a well-controlled study in support of a new indication for use or not intended to be used to support any other significant change in the labeling for the drug; (ii) If the drug that is undergoing investigation is lawfully marketed as a prescription drug product, the investigation is not intended to support a significant change in the advertising for the drug;

When Do I Need an IND? CFR 312. 2 (b) Exemptions (continued) (iii) The investigation does not involve a route of administration or dosage level or use in a patient population or other factor that significantly increases the risks (or decreases the acceptability of the risks) associated with the use of the drug product; (iv) The investigation is conducted in compliance with the requirements for institutional review set forth in part 56 and with the requirements for informed consent set forth in part 50; and (v) The investigation is conducted in compliance with the requirements of CFR 312. 7 (Promotion and charging for investigational drugs).

Who Do I Contact to Find Out if I Need an IND? Contacts: Drugs: Biologics: Devices: Foods: Barry Poole Robert Yetter IDE Staff David Hattan (301) 827 -3454 (301) 827 -0373 (240) 276 -0125 (202) 436 -1293 Call, and then submit the request in writing (FAX or email). FDA plans to issue a guidance for this question.

DSI’s BIMO Program Responsibilities g Good Clinical Practice (GCP) n Clinical Investigators n Sponsor-Monitors, CROs n Human Subject Protection Program (IRB) g Good Laboratory Practice (GLP) g In vivo Bioequivalence

GCP BIMO Program Clinical Investigator Inspection Program n Sponsor/Monitor/CRO Inspection Program n These two programs collectively allow the agency to determine: n n n Adherence to applicable regulations Validity of studies in support of pending marketing applications Whether the rights and safety of subjects have been protected

DSI’s BIMO Responsibilities g DSI is responsible for all BIMO inspections for CDER n Developing assignments, often in consultation with CDER review divisions n Issuing assignments to Office of Regulatory Affairs (ORA) field investigators and participating on inspection when scientific or medical expertise is required n Evaluating the results of inspections from a scientific and regulatory perspective.

DSI’s BIMO Responsibilities g DSI is responsible for all BIMO inspections for CDER n Recommending scientific follow-up n Recommending and implementing regulatory actions n Providing expert advice on program design, policy issues and guidance n Educating and informing program constituents

What we do in the DSI GCP Branches For the CDER Review Divisions DSI will arrange for routine data audit GCP inspections to determine data integrity and safety of subjects in pivotal clinical trials, and provide the inspection reports to the review division prior to the Division Action Goal Date For the Public DSI will investigate complaints related to the conduct of clinical trials, including arranging for directed or “for cause” GCP inspections, and take appropriate regulatory action.
About BIMO Inspections n BIMO inspections can be conducted at any point in the drug development process n Inspections during IND phase are generally “for cause = directed” n Inspections during the NDA phase are generally “routine”, but can be “for cause” or “directed” n May include Clinical Investigator (CI), Sponsor/Monitoring (S/M), Contract Research Organizations (CRO), Institutional Review Boards (IRB), Good Laboratory Practice (GLP), and Bioequivalence (BEq) inspection of FDA regulated research.
About BIMO Inspections Routine n Inspections assigned for NDAs Directed (“for cause”) n n Problems identified during FDA review process Complaints reported to DSI from n FDA, other Agencies n Sponsors/monitors n Institutions/IRB’s n Site personnel n Subjects/Public

Clinical Investigator Responsibilities* n n n Follow the current protocol Personally conduct or supervise investigation(s) Ensure that all persons assisting in conduct of studies are informed of their obligations. Ensure informed consent (21 CFR 50) and IRB review, approval , and reporting (21 CFR 56) requirements are met. Obtain the informed consent of each human subject to whom the drug is administered. *(Form FDA 1572: #9. Commitments)

Clinical Investigator Responsibilities* n n n Notify the sponsor before making changes in the protocol. Notify the IRB and obtain IRB approval before making changes in the protocol. Report adverse events to the sponsor. Maintain adequate and accurate records. Make records available for inspection. Comply with all other requirements in 21 CFR 312. *(Form FDA 1572: #9. Commitments)

Clinical Investigator Inspections What do we look for during the inspection? The FDA Inspection (Audit) compares ± Source Medical Record Data vs ± Case Report Forms vs ± Data Listing Submitted to NDA

Clinical Investigator Inspections What do we look for during the inspection? n Clinical Investigator inspection determines ± Source of subjects ± Did subjects exist? ± Did they have the disease under study? ± Did they meet inclusion/exclusion criteria? ± Consent obtained? ± IRB Review Obtained?

Clinical Investigator Inspections What do we look for during the inspection? n Clinical Investigator inspection determines ± Was the protocol followed? ± Did the subjects receive the assigned study drug in the dose, route and frequency specified by the protocol? ± Are the case report forms complete and in agreement with source data? Compare with NDA data listing ± Are adverse experiences reported to sponsor and IRB? ± Adequacy and completeness of records?

Clinical Investigator Inspections All Centers - 2004 CDER: n CBER: n CDRH: 351 71 178 Total: 600 n n

Criteria for Assigning International Inspections International sites may be audited n if there are insufficient domestic data; n only foreign data are submitted to support an application; n domestic and foreign data show conflicting results pertinent to decision-making; or n there is a serious issue to resolve, e. g. , suspicion of fraud, scientific misconduct, significant human subject protection violations.
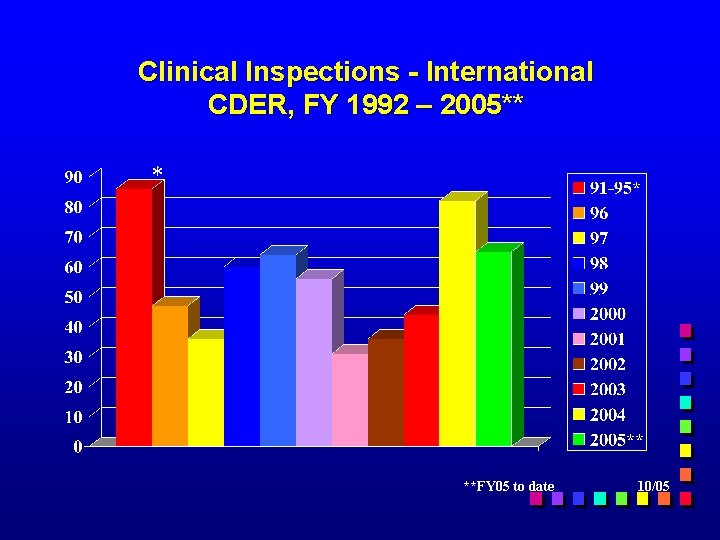
Clinical Inspections - International CDER, FY 1992 – 2005** * **FY 05 to date 10/05
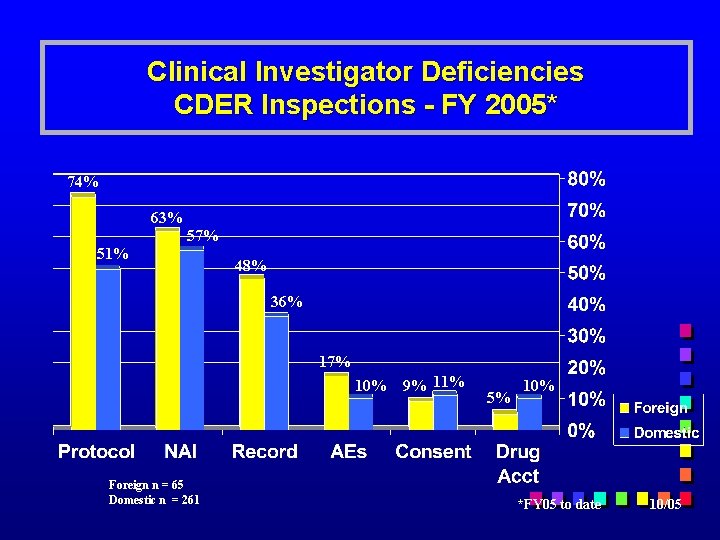
Clinical Investigator Deficiencies CDER Inspections - FY 2005* 74% 63% 57% 51% 48% 36% 17% 10% Foreign n = 65 Domestic n = 261 9% 11% 5% 10% *FY 05 to date 10/05
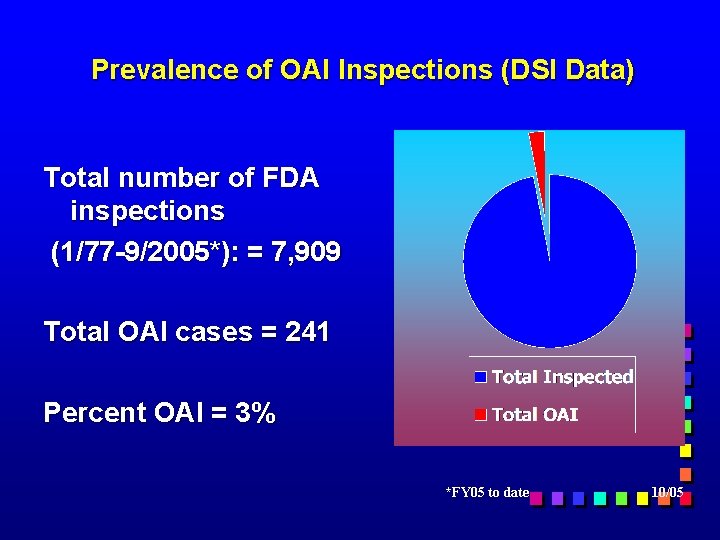
Prevalence of OAI Inspections (DSI Data) Total number of FDA inspections (1/77 -9/2005*): = 7, 909 Total OAI cases = 241 Percent OAI = 3% *FY 05 to date 10/05
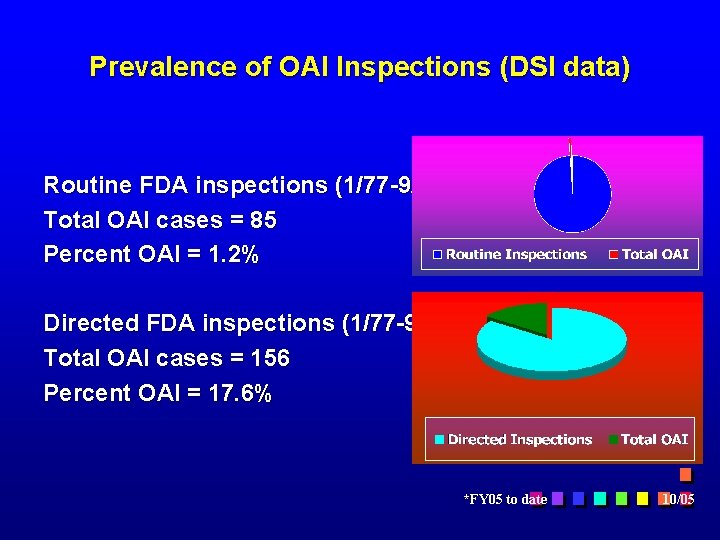
Prevalence of OAI Inspections (DSI data) Routine FDA inspections (1/77 -9/2005*) = 7023 Total OAI cases = 85 Percent OAI = 1. 2% Directed FDA inspections (1/77 -9/2005*) = 886 Total OAI cases = 156 Percent OAI = 17. 6% *FY 05 to date 10/05

Complaints to DSI The number of complaints about clinical investigators/clinical trials continues to increase n DSI encourages such reporting (new Electronic Complaint Form) n Follow-up on complaints is of vital strategic importance n n Real-time follow-up of real-time issues n Public protection; Public confidence

What Are They Complaining About? n n n n Informed Consent Issues Falsification Failure to report adverse events Failure to follow the protocol Inadequate Records Qualifications of persons performing physicals Failure to get IRB approval, report changes in research Failure to follow FDA regulations n n n n n Drug accountability Recruitment Practices Poor Supervision No active IND Violations of GLP regs Monitoring practices Blinding Charging for the test article Misleading advertisements
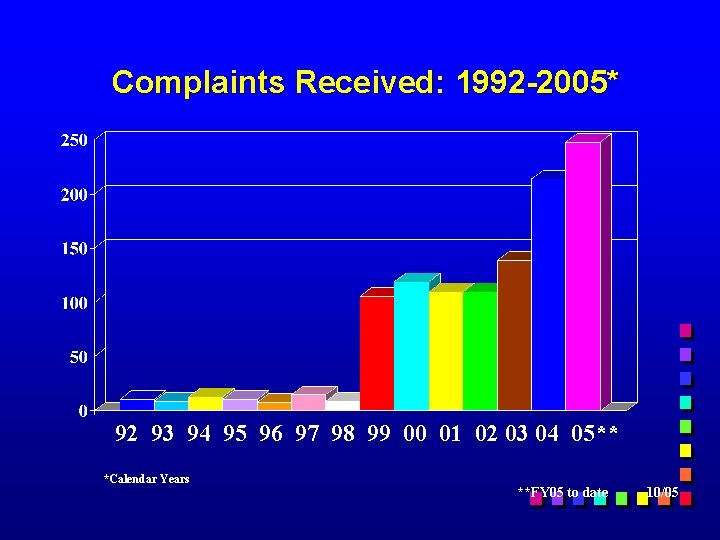
Complaints Received: 1992 -2005* 92 93 94 95 96 97 98 99 00 01 02 03 04 05** *Calendar Years **FY 05 to date 10/05
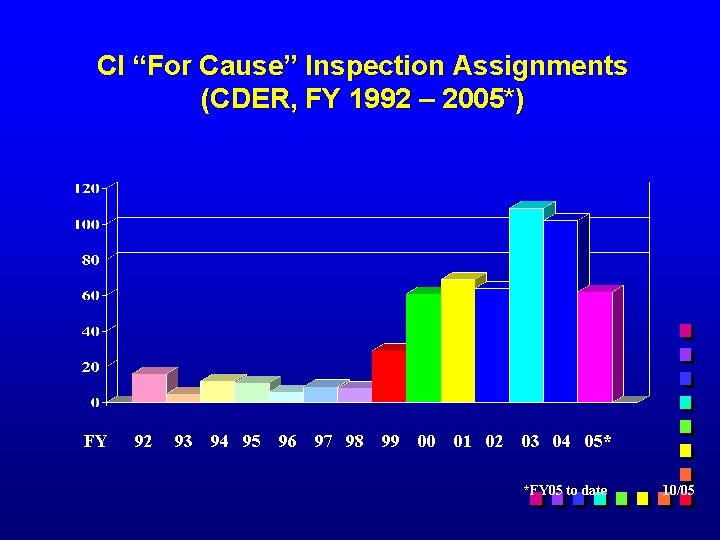
CI “For Cause” Inspection Assignments (CDER, FY 1992 – 2005*) FY 92 93 94 95 96 97 98 99 00 01 02 03 04 05* *FY 05 to date 10/05

Sponsor Responsibilities n A sponsor shall not begin a clinical investigation until the IND is in effect (21 CFR 312. 20), which means: n after notification by FDA to begin investigation n OR n 30 days after FDA receives the IND n A sponsor shall not begin a clinical investigation if FDA places the study on clinical hold, which is an order to delay or suspend an investigation (21 CFR 312. 40)

Sponsor Responsibilities n 21 CFR 312. 50 - General responsibilities of sponsors n Selecting qualified investigators n Providing investigators with the information they need to conduct an investigation properly (e. g. , protocol, IB, safety reports) n Ensure proper monitoring of the investigation

Sponsor Responsibilities n 21 CFR 312. 50 - General responsibilities of sponsors n Ensure that the investigation is conducted in accordance with the investigational plan n Maintain an effective IND (e. g. , annual reports, safety reports, amendments, …) n Ensure that FDA and investigators are promptly informed of significant new adverse effects or risks (e. g. , safety reports)

21 CFR 312. 53 -Selecting investigators and monitors Sponsors shall select only investigators qualified by training and experience as appropriate experts to investigate the drug (b) Sponsors shall only ship drug to participating investigators (c) Sponsors shall obtain 1572, a statement of qualifications, and financial disclosure info. (d) Sponsors shall select qualified monitors (a)

Human Subject Protection Program IRB inspection program n Consults from review divisions n Inquiries from IRBs and public n

Institutional Review Board (IRB) Formally designated by an institution to review, approve initiation of, and conduct periodic review of biomedical research involving human subjects n Primary purpose is to assure the protection of the rights and welfare of the human subjects n Scientific considerations included but not primary n

IRB Inspections: What’s done? n n Purpose: to assess compliance with 21 CFR Part 56 IRB and study-specific records reviewed n Written procedures n n n Initial and continuing review Reporting findings and actions to CI and institution Ensuring prompt reporting of n n n n Research changes Unanticipated problems involving risk Serious or continuing non-compliance Suspension or termination of IRB approval Meeting minutes: attendance, actions taken, votes, basis for requiring changes or disapproval, controverted issues and resolution Continuing review activities All correspondence w/CI Membership list(s)

IRB Inspections: What’s done? n FDA-regulated studies are selected Criteria for IRB approval (risks minimized, reasonable; equitable subject selection; informed consent; compliance with Part 50, Subpart D as appropriate; add’l safeguards for other vulnerable populations) n Documentation related to study approvals, continuing review, suspensions/terminations, unanticipated problems involving risk to subjects or others n Informed consent documents n Use of expedited review n
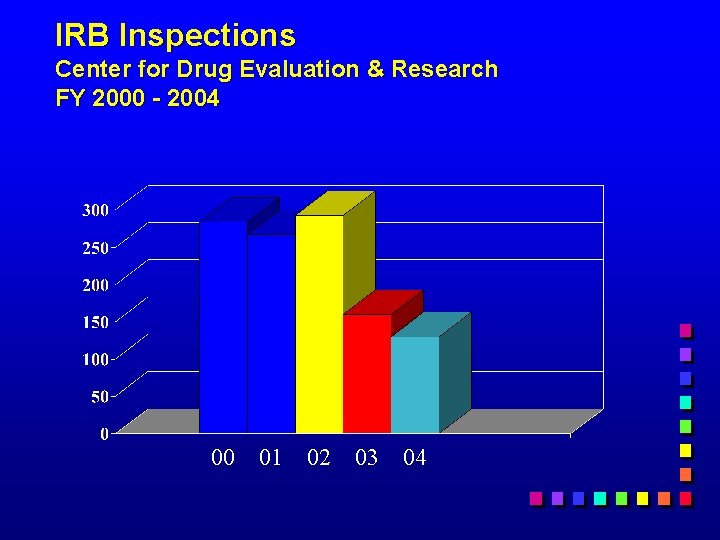
IRB Inspections Center for Drug Evaluation & Research FY 2000 - 2004 00 01 02 03 04
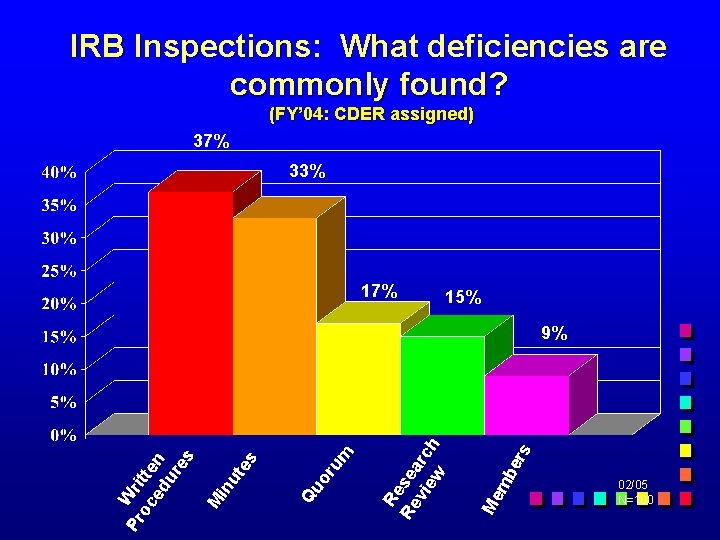
IRB Inspections: What deficiencies are commonly found? (FY’ 04: CDER assigned) 37% 33% 17% 15% ers mb Me Re Re sea vi rc ew h um or Qu Pr Wri oc tte ed n ur es M in ut es 9% 02/05 N=100

IRB inspections: Perspective n Major differences among IRBs: n Local vs. central n Expertise and number of IRB members n Number of studies reviewed n Type of studies reviewed n Potential risk to human subjects n Trial population

Evaluation of IRBs Friend, not Foe n FDA has traditionally viewed IRBs as allies in human subject protection n Official Action (OAI) is a last resort

What is Generated After an Inspection Form FDA 483: Inspectional Observations n Left with inspected party at close of inspection n Immediately available under the Freedom of Information Act Establishment Inspection Report (EIR) n Prepared by field investigator after inspection n Includes exhibits supporting observed deficiencies

What is Generated After an Inspection DSI Review of EIR n Point-by-point review of 483 items, additional deficiencies, limitations of inspection n Confirmation of supporting documentation n Assessment of any response from the inspected party n For GCP inspections, often include assessment of impact on acceptability of data for the drug approval review n Final compliance classification

Compliance Classifications NAI - No Action Indicated n Firm is in compliance VAI - Voluntary Action Indicated Minor deviation(s) from the regulations n Voluntary correction requested n OAI - Official Action Indicated n Serious non-compliance requiring regulatory or administrative action by FDA; Data Unacceptable
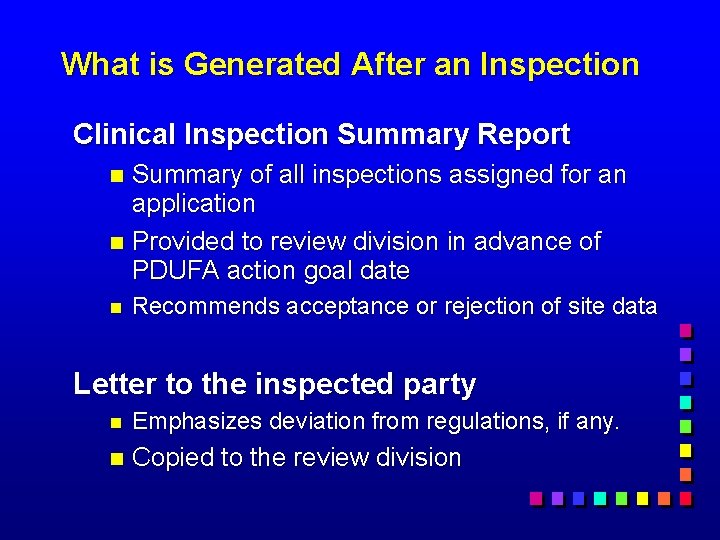
What is Generated After an Inspection Clinical Inspection Summary Report Summary of all inspections assigned for an application n Provided to review division in advance of PDUFA action goal date n n Recommends acceptance or rejection of site data Letter to the inspected party n Emphasizes deviation from regulations, if any. n Copied to the review division

What is Generated After an Inspection Where Official Action is Indicated n n Recommendation to Reject Data n enforcement option for non-U. S. , non-IND sites Warning Letter Disqualification of a clinical investigator (21 CFR 312. 70) n Notice of Initiation of Disqualification Proceedings and Opportunity to Explain (NIDPOE) letter n Informal Conference n Notice of Opportunity for Hearing (Formal) n Consent Agreement n Part 16 Disqualification Hearing Referral for criminal prosecution (OCI)
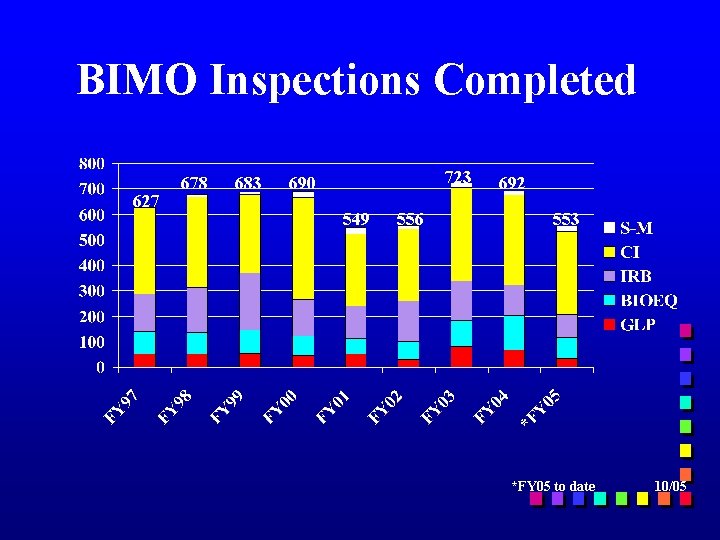
BIMO Inspections Completed 627 678 683 723 690 549 556 692 553 *FY 05 to date 10/05
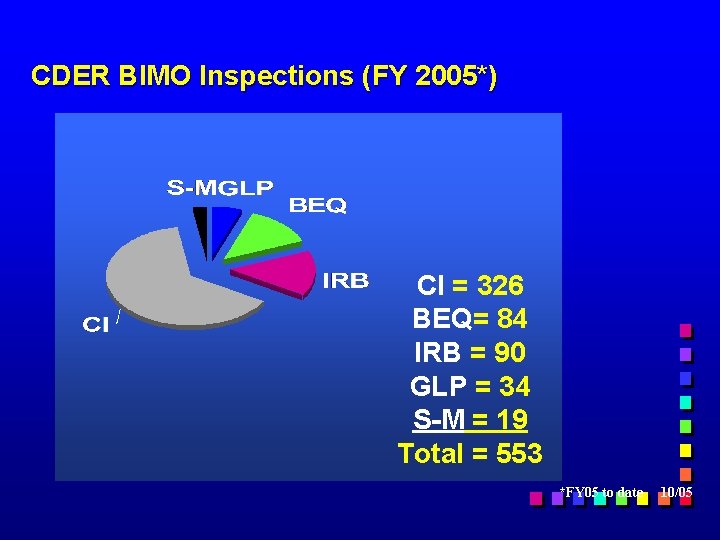
CDER BIMO Inspections (FY 2005*) CI = 326 BEQ= 84 IRB = 90 GLP = 34 S-M = 19 Total = 553 *FY 05 to date 10/05

Helpful Websites n DSI Homepage: www. fda. gov/cder/offices/dsi Includes links to the Clinical Investigator Inspection List (NEW), Bioresearch Monitoring Information Systems (BMIS) files (NEW), Warning Letters, NIDPOE Letters, Lists of Disqualified or Restricted or Debarred Investigators, Code of Federal Regulations, etc. n FDA Homepage: www. fda. gov Includes links to the Federal Register Notices, FDA guidance documents. n Compliance Programs: www. fda. gov/ora/compliance_ref/default. htm
- Slides: 52