FDA Food Safety Modernization Act Michael Rogers M





















- Slides: 25

FDA Food Safety Modernization Act Michael Rogers, M. S. Director, Latin America Office Food and Drug Administration FDA – Latin America Regional Office US-FDA-LAO@fda. hhs. gov 9/30/2020 #1 FDA’s Current Thinking: Proposed Produce Safety Regulation

Agenda • Why is the law needed? • Provisions of the law; focus on imports • Implementation 9/30/2020 #2 FDA’s Current Thinking: Proposed Produce Safety Regulation

New law updates authority and tools 2011 – Food Safety Modernization Act 1976 Medical Device Amendments 1938 – Food, Drug, and Cosmetic Act 1906 – Pure Food and Drug Act 9/30/2020 #3 FDA’s Current Thinking: Proposed Produce Safety Regulation

Why is the law needed? Globalization • 15 percent of U. S. food supply is imported Food supply more high-tech & complex • More foods in the marketplace • New hazards in foods not previously seen Shifting demographics • Growing population (about 30%) of individuals are especially “at risk” for foodborne illness 9/30/2020 #4 FDA’s Current Thinking: Proposed Produce Safety Regulation

The Public Health Imperative • Foodborne illness is a significant burden – About 48 million (1 in 6 Americans) get sick each year – 128, 000 are hospitalized – 3, 000 die • Immune-compromised individuals more susceptible – Infants and children, pregnant women, older individuals, those on chemotherapy • Foodborne illness is not just a stomach ache—it can cause life-long chronic disease – Arthritis, kidney failure 9/30/2020 #5 FDA’s Current Thinking: Proposed Produce Safety Regulation

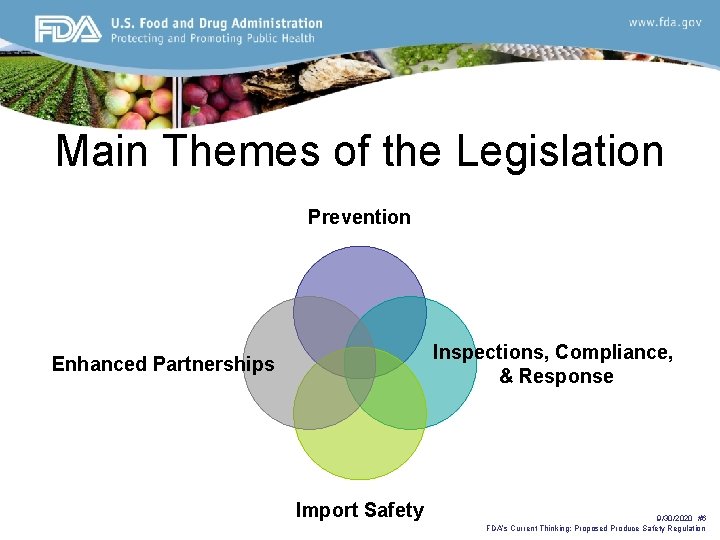
Main Themes of the Legislation Prevention Inspections, Compliance, & Response Enhanced Partnerships Import Safety 9/30/2020 #6 FDA’s Current Thinking: Proposed Produce Safety Regulation

Prevention: The cornerstone of the legislation • Comprehensive preventive controls for food facilities – Prevention is not new, but Congress gave FDA explicit authority to use the tool more broadly – Strengthens accountability for prevention • Produce safety standards • Intentional adulteration standards 9/30/2020 #7 FDA’s Current Thinking: Proposed Produce Safety Regulation

Inspection, Compliance & Response • Mandated inspection frequency – Considering new ways to inspect • New tools – – – Mandatory recall Expanded records access Expanded administrative detention Suspension of registration Enhanced product tracing Third party laboratory testing 9/30/2020 #8 FDA’s Current Thinking: Proposed Produce Safety Regulation

Enhanced Partnerships: Vital to Success • Reliance on inspections by other agencies that meet standards • State/local & international capacity building • Improve foodborne illness surveillance • National agriculture & food defense strategy • Consortium of laboratory networks • Easier to find recall information 9/30/2020 #9 FDA’s Current Thinking: Proposed Produce Safety Regulation

Import Safety: Most Groundbreaking Shift • Importers now responsible for ensuring their suppliers have adequate preventive controls in place • Can rely on third parties to certify that foreign food facilities meet U. S. requirements • Can require mandatory certification for high-risk foods • Voluntary qualified importer program--expedited review • Can deny entry if FDA access for inspection is denied • Requires food from abroad to be as safe as domestic 9/30/2020 #10 FDA’s Current Thinking: Proposed Produce Safety Regulation

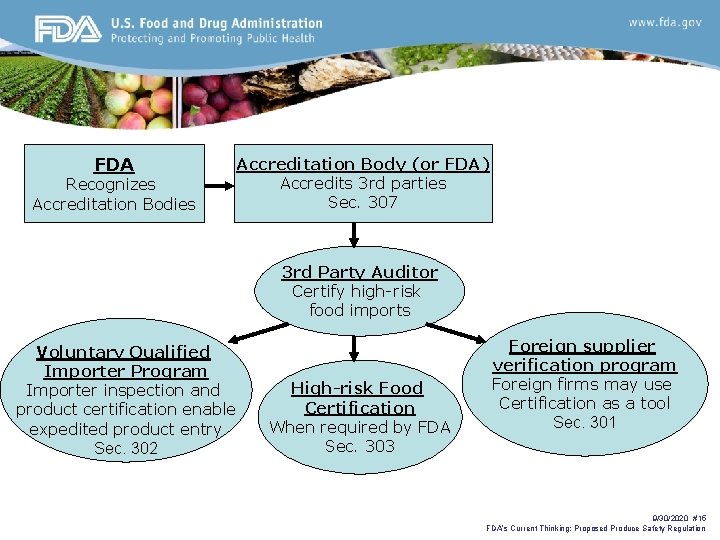
Import Safety Mandates Sec. 301. Foreign supplier verification program • Requires importers to verify their suppliers use riskbased preventive controls that provide same level of protection as U. S. requirements and that product is not adulterated or misbranded. Sec. 302. Voluntary qualified importer program • Allows for expedited review and entry of products from qualified importers received from certified facilities Sec. 303. Certification for high-risk food imports • FDA has discretionary authority to require assurances of compliance for high-risk foods 9/30/2020 #11 FDA’s Current Thinking: Proposed Produce Safety Regulation

Import Safety Mandates Sec. 304. Prior notice of imported food shipments • Requires information on prior refusals to be added to prior notice submission Sec. 305. Capacity building • FDA mandate to work with foreign governments to build food safety capacity Sec. 306. Inspection of foreign food facilities • Can deny entry if FDA access for inspection is denied Sec. 201. Targeting of inspection resources • Increased inspection of foreign as well as domestic facilities 9/30/2020 #12 FDA’s Current Thinking: Proposed Produce Safety Regulation

Import Safety Mandates Sec. 307. Accreditation of third-party auditors • FDA can rely on accredited third parties to certify that foreign food facilities meet U. S. requirements Sec. 308. Foreign Offices of the Food and Drug Administration. • Establish offices in foreign countries to provide assistance on food safety measures for food exported to the U. S. Sec. 309. Smuggled Food • In coordination with DHS, better identify and prevent entry of smuggled food 9/30/2020 #13 FDA’s Current Thinking: Proposed Produce Safety Regulation

Role of Third-Party Certification Programs • Tool for importers to obtain needed assurances to meet their obligations for the foreign supplier verification program (sec. 301) • A way for importers to participate in the voluntary qualified importer program to expedite movement of food through the import process (sec. 302) • Can be required by FDA to accompany high-risk foods (sec. 303) 9/30/2020 #14 FDA’s Current Thinking: Proposed Produce Safety Regulation

FDA Recognizes Accreditation Bodies Accreditation Body (or FDA) Accredits 3 rd parties Sec. 307 3 rd Party Auditor Certify high-risk food imports Voluntary Qualified Importer Program Importer inspection and product certification enable expedited product entry Sec. 302 High-risk Food Certification When required by FDA Sec. 303 Foreign supplier verification program Foreign firms may use Certification as a tool Sec. 301 9/30/2020 #15 FDA’s Current Thinking: Proposed Produce Safety Regulation

Implementation Approach • • • Implementation already underway Coalition needed Transparency a priority Focus on public health protection Engage with stakeholders to help determine reasonable and practical ways to implement provisions 9/30/2020 #16 FDA’s Current Thinking: Proposed Produce Safety Regulation

Triggers • • • Legislation Petition Court Decision Accident/Incident Technology Triggering Event FSMA Regulatory Process (Rulemaking) Proposed Rule Final Rule Effective Date (Step 1) (Step 2) (Step 3) Initial Research • Identify problem • Substantiate problem • Determine solution WE ARE HERE Additional Tools 9/30/2020 #17 FDA’s Current Thinking: Proposed Produce Safety Regulation

Rulemaking Process: It Doesn’t Happen Overnight 1. FDA proposes rule and requests comments We are nearly here 2. FDA considers comments and issues final rule 3. FDA sets dates for companies to comply 9/30/2020 #18 FDA’s Current Thinking: Proposed Produce Safety Regulation

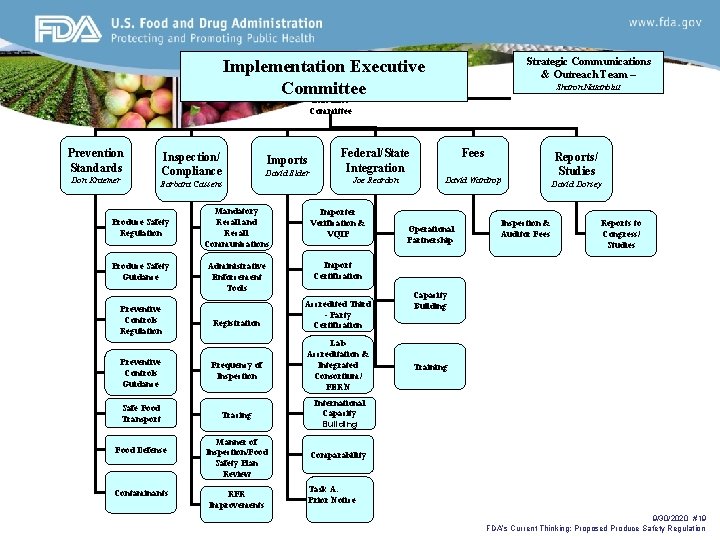
Strategic Communications & Outreach Team – Implementation Executive Implementation Committee Sharon Natanblut Executive Committee Prevention Standards Don Kraemer Inspection/ Compliance Imports David Elder Barbara Cassens Produce Safety Regulation Produce Safety Guidance Preventive Controls Regulation Federal/State Integration Fees Joe Reardon David Wardrop Mandatory Recall and Recall Communications Importer Verification & VQIP Administrative Enforcement Tools Import Certification Registration Accredited Third - Party Certification Lab Accreditation & Integrated Consortium/ FERN Preventive Controls Guidance Frequency of Inspection Safe Food Transport Tracing International Capacity Building Manner of Inspection/Food Safety Plan Review Comparability Food Defense Contaminants RFR Improvements Operational Partnership Inspection & Auditor Fees Reports/ Studies David Dorsey Reports to Congress/ Studies Capacity Building Training Task A: Prior Notice 9/30/2020 #19 FDA’s Current Thinking: Proposed Produce Safety Regulation

Implementation & Compliance Educate before we regulate • Partner with stakeholders to provide education & outreach Non-traditional strategy: • Educate & outreach to enhance compliance • Small entity compliance guide on how to comply with the regulations • Updated GAPs guidance • • Utilize existing & develop new partnerships with governments Consider how existing efforts & information may be used Develop appropriate review & oversight mechanism Interface with trade associations, commodity groups, individuals with diverse farming practices and operations Flexibility built into regulation via Alternative approaches, Variances and Compliance dates 9/30/2020 #20 FDA’s Current Thinking: Proposed Produce Safety Regulation

Additional Resources • FDA FSMA page: http: //www. fda. gov/Food. Safety/FSMA/default. htm • Produce Safety Alliance: http: //producesafetyalliance. cornell. edu/psa. html • FDA Produce Safety Activities: http: //www. fda. gov/Food. Safety/Product-Specific. Information/ Fruits. Vegetables. Juices/FDAProduce. Safety. Activities/default. htm 9/30/2020 #21 FDA’s Current Thinking: Proposed Produce Safety Regulation

For more information • Web site at: www. fda. gov/fsma • Subscription feature available To Submit Comments: • www. regulations. g ov 9/30/2020 #22 FDA’s Current Thinking: Proposed Produce Safety Regulation

9/30/2020 #23 FDA’s Current Thinking: Proposed Produce Safety Regulation

Thank you! Questions? us-fda-lao@fda. hhs. gov • San José, CR: (506) 2519 -2224 • México DF : (52) (55) 1997 -1506 • Santiago, Chile: (562) 330 3035 9/30/2020 #24 FDA’s Current Thinking: Proposed Produce Safety Regulation

• References: • www. slideshare. net 9/30/2020 #25 FDA’s Current Thinking: Proposed Produce Safety Regulation