FDA Case for Quality Beth Staub and Joseph

FDA Case for Quality Beth Staub and Joseph Sapiente 1

The Medical Device Industry • 2 2 Projected $133 B 2016 • More than 6, 500 companies • 80% less than 50 people

The Case for Quality Began the dialogue: • Focus on Compliance vs Product Quality • Measurement and Transparency • Culture 3 3

The Case for Quality “ the regulation does not prescribe in detail how a manufacturer must produce a specific device. Rather, the regulation provides the framework that all manufacturers must follow by requiring that manufacturers develop and follow procedures and fill in the details” The Regulations Provide the “What” 4 4

The “WHAT” of CAPA PART 820. 100 - Corrective and Preventive action: • Establish and maintain procedures… • Investigating the cause of nonconformities… • Identifying the action needed… • Verifying or validating the action … • Implementing and recording changes …… 5 5

The “HOW” of CAPA • Containment and Interim Corrective Action • Problem Solving - DMAIC, 8 D, 5 Whys, Fishbone diagrams • Project Management • Metrics • Culture - Reward and Recognition 6 6

The Questions We are all Asking “I don’t know exactly what product quality even means” “OK, thanks for telling me what it means…how do I recognize it, measure it, know if I have it? ” “Daunting! How do I figure out the practical ‘know how’ to implement this well in my company? ” “How can we help the business people appreciate the value of quality so they will support our efforts? ” “Is FDA going to support this approach? ” 7 7

Case for Quality Framework Initiated from FDA’s 2012 Whitepaper: “Understanding the Barriers to Device Quality” FDA QSR 21. CFR. 820 Local Regulations Product Quality Definition How Evolve Culture from Compliance to Quality How to Derive the Value ISO 13485 8 8 Standards How to Implement How to Measure
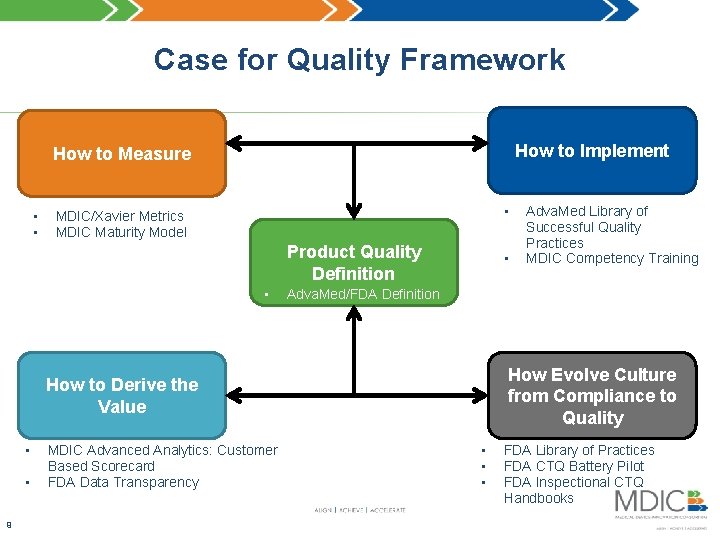
Case for Quality Framework How to Implement How to Measure • • • MDIC/Xavier Metrics MDIC Maturity Model Product Quality Definition • • Adva. Med/FDA Definition How Evolve Culture from Compliance to Quality How to Derive the Value • • 9 9 MDIC Advanced Analytics: Customer Based Scorecard FDA Data Transparency Adva. Med Library of Successful Quality Practices MDIC Competency Training • • • FDA Library of Practices FDA CTQ Battery Pilot FDA Inspectional CTQ Handbooks

Your Input is Needed As you listen and interact today… • What are your reactions? − Framework − Projects − Approach to working together • What could be added, adjusted, re-prioritized? • Any other suggestions? • Any way you want to be more involved? 10 10
- Slides: 10