Fatty acid Catabolism oxidation Beta Oxidation of Fatty

Fatty acid Catabolism ( -oxidation)

Beta Oxidation of Fatty Acids • Process by which fatty acids are degraded by removal of 2 -C units • -oxidation occurs in the mitochondria matrix • The 2 -C units are released as acetyl. Co. A, not free acetate • The process begins with oxidation of the carbon that is "beta" to the carboxyl carbon, so the process is called"betaoxidation"

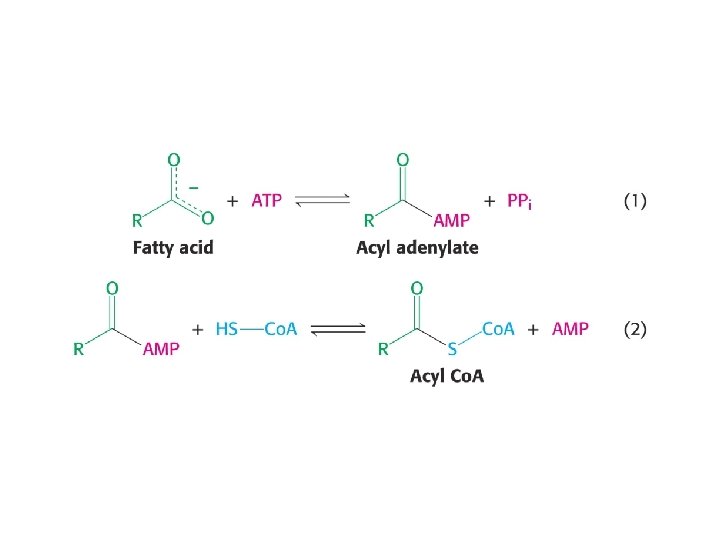
Fatty acids must first be activated by formation of acyl-Co. A • Acyl-Co. A synthetase condenses fatty acids with Co. A, with simultaneous hydrolysis of ATP to AMP and PPi • Formation of a Co. A ester is expensive energetically • Reaction just barely breaks even with ATP hydrolysis Go’ATP hydroysis = -32. 3 k. J/mol, Go’ Acyl-Co. A synthesis +31. 5 k. J/mol. • But subsequent hydrolysis of PPi drives the reaction strongly forward ( Go’ – 33. 6 k. J/mol)
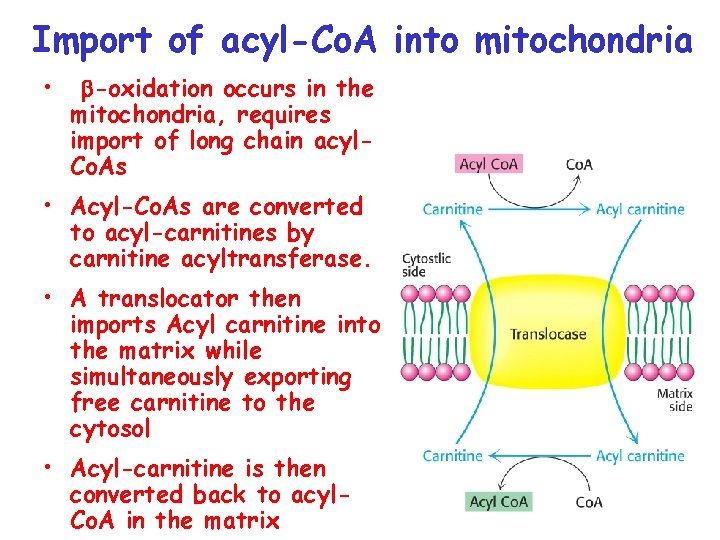
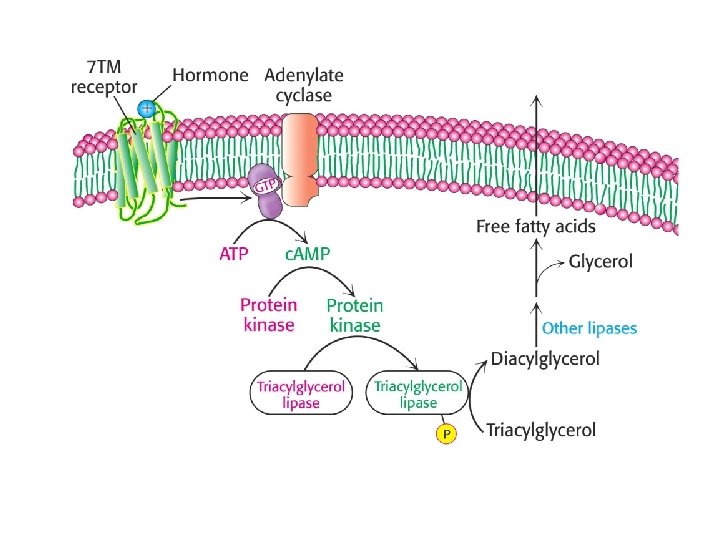
Import of acyl-Co. A into mitochondria • -oxidation occurs in the mitochondria, requires import of long chain acyl. Co. As • Acyl-Co. As are converted to acyl-carnitines by carnitine acyltransferase. • A translocator then imports Acyl carnitine into the matrix while simultaneously exporting free carnitine to the cytosol • Acyl-carnitine is then converted back to acyl. Co. A in the matrix
Deficiencies of carnitine or carnitine transferase or translocator activity are related to disease state • Symptons include muscle cramping during exercise, severe weakness and death. • Affects muscles, kidney, and heart tissues. • Muscle weakness related to importance of fatty acids as long term energy source • People with this disease supplement diet with medium chain fatty acids that do not require carnitine shuttle to enter mitochondria.
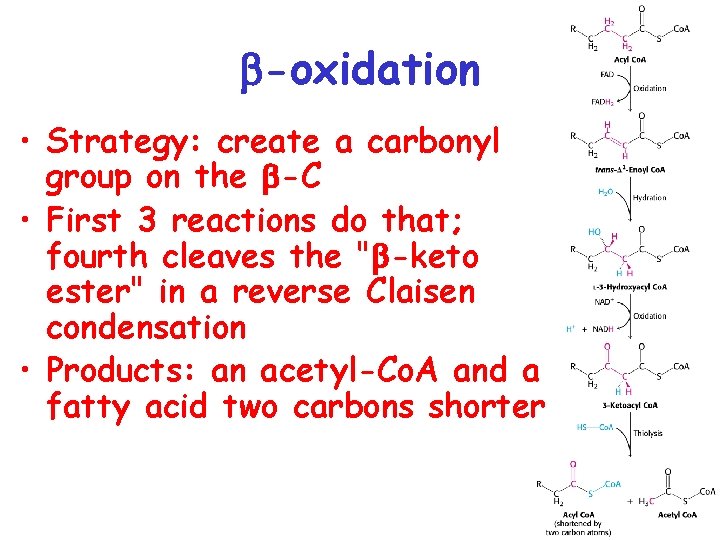
-oxidation • Strategy: create a carbonyl group on the -C • First 3 reactions do that; fourth cleaves the " -keto ester" in a reverse Claisen condensation • Products: an acetyl-Co. A and a fatty acid two carbons shorter
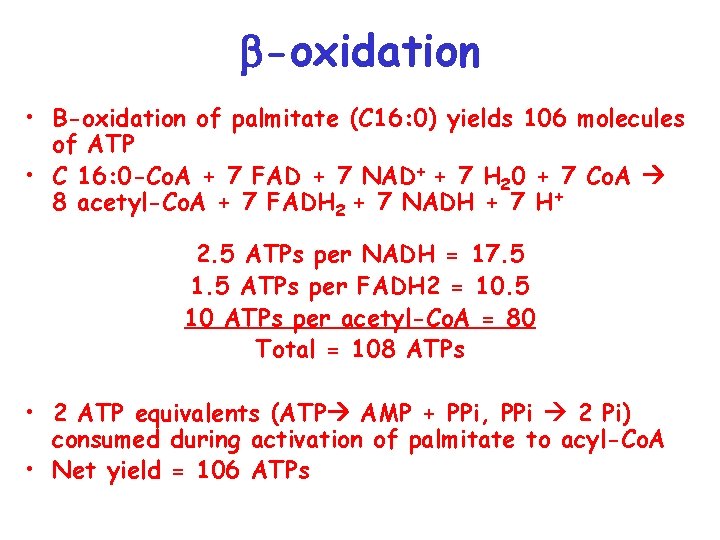
-oxidation • B-oxidation of palmitate (C 16: 0) yields 106 molecules of ATP • C 16: 0 -Co. A + 7 FAD + 7 NAD+ + 7 H 20 + 7 Co. A 8 acetyl-Co. A + 7 FADH 2 + 7 NADH + 7 H+ 2. 5 ATPs per NADH = 17. 5 1. 5 ATPs per FADH 2 = 10. 5 10 ATPs per acetyl-Co. A = 80 Total = 108 ATPs • 2 ATP equivalents (ATP AMP + PPi, PPi 2 Pi) consumed during activation of palmitate to acyl-Co. A • Net yield = 106 ATPs
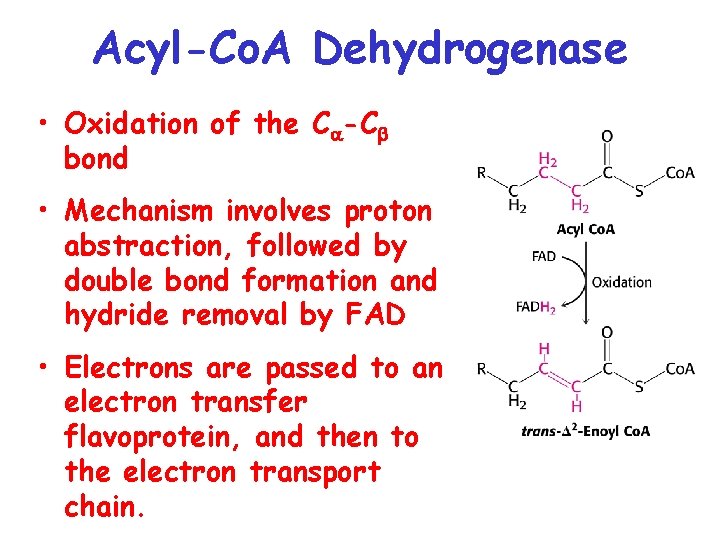
Acyl-Co. A Dehydrogenase • Oxidation of the C -C bond • Mechanism involves proton abstraction, followed by double bond formation and hydride removal by FAD • Electrons are passed to an electron transfer flavoprotein, and then to the electron transport chain.
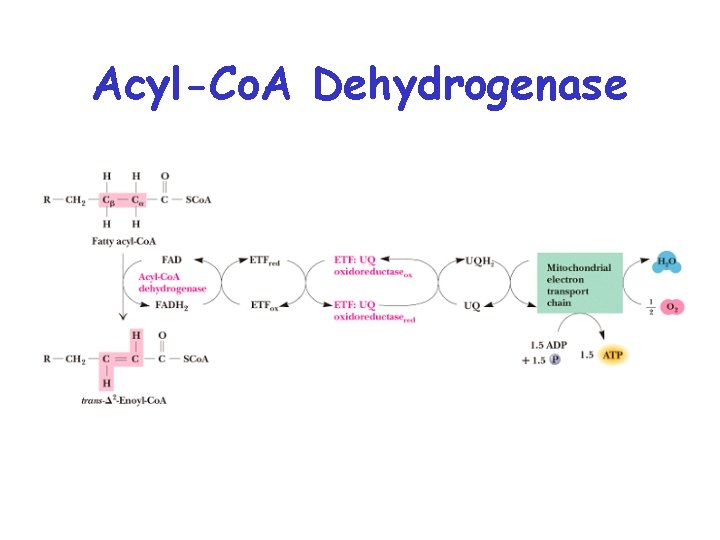
Acyl-Co. A Dehydrogenase
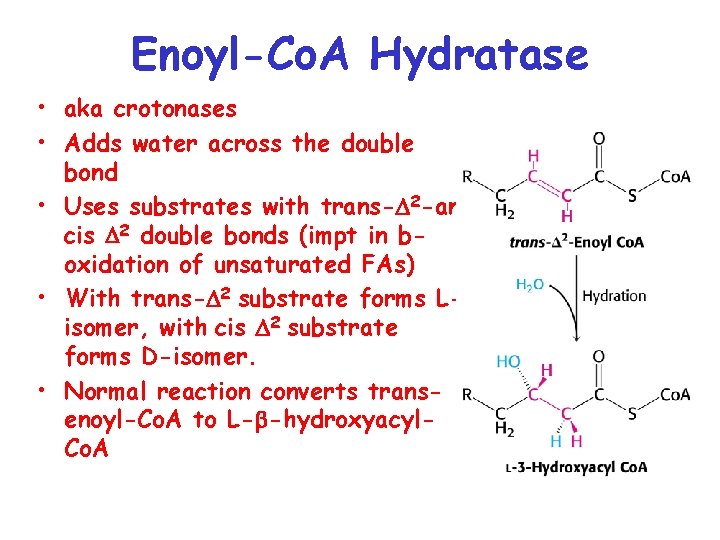
Enoyl-Co. A Hydratase • aka crotonases • Adds water across the double bond • Uses substrates with trans- 2 -and cis 2 double bonds (impt in boxidation of unsaturated FAs) • With trans- 2 substrate forms Lisomer, with cis 2 substrate forms D-isomer. • Normal reaction converts transenoyl-Co. A to L- -hydroxyacyl. Co. A
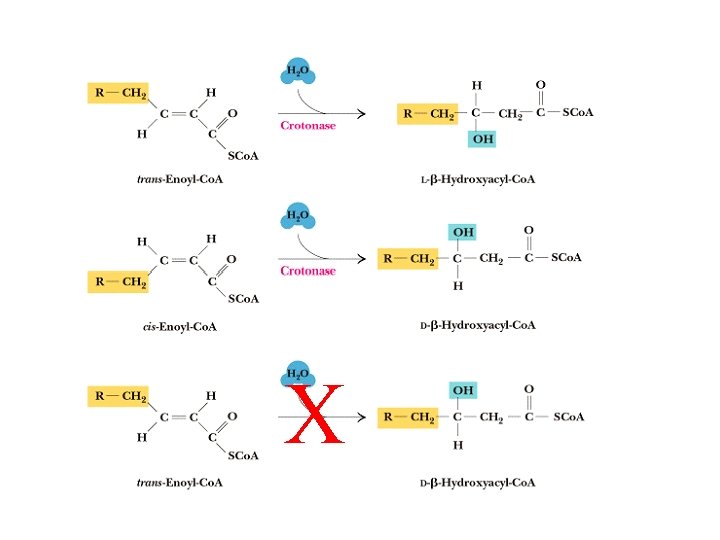
X
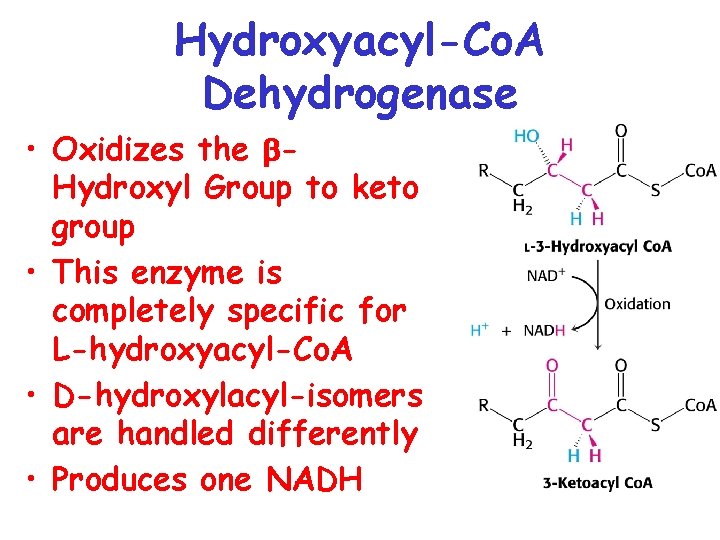
Hydroxyacyl-Co. A Dehydrogenase • Oxidizes the Hydroxyl Group to keto group • This enzyme is completely specific for L-hydroxyacyl-Co. A • D-hydroxylacyl-isomers are handled differently • Produces one NADH
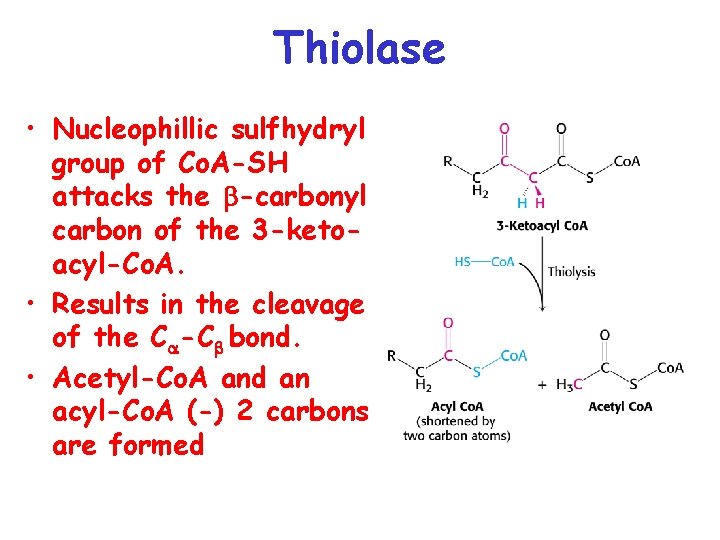
Thiolase • Nucleophillic sulfhydryl group of Co. A-SH attacks the -carbonyl carbon of the 3 -ketoacyl-Co. A. • Results in the cleavage of the C -C bond. • Acetyl-Co. A and an acyl-Co. A (-) 2 carbons are formed
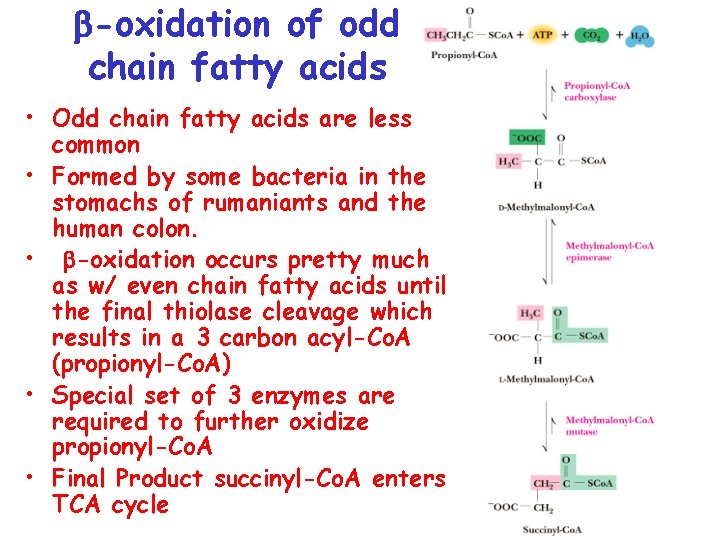
-oxidation of odd chain fatty acids • Odd chain fatty acids are less common • Formed by some bacteria in the stomachs of rumaniants and the human colon. • -oxidation occurs pretty much as w/ even chain fatty acids until the final thiolase cleavage which results in a 3 carbon acyl-Co. A (propionyl-Co. A) • Special set of 3 enzymes are required to further oxidize propionyl-Co. A • Final Product succinyl-Co. A enters TCA cycle

-oxidation of unsaturated fatty acids • • • -oxidation occurs normally for 3 rounds until a cis- 3 -enoyl-Co. A is formed. Acyl-Co. A dehydrogenase can not add double bond between the and carbons. Enoyl-Co. A isomerase converts this to trans- 2 enoly-Co. A Now the -oxidation can continue on w/ the hydration of the trans 2 -enoyl-Co. A Odd numbered double bonds handled by isomerase
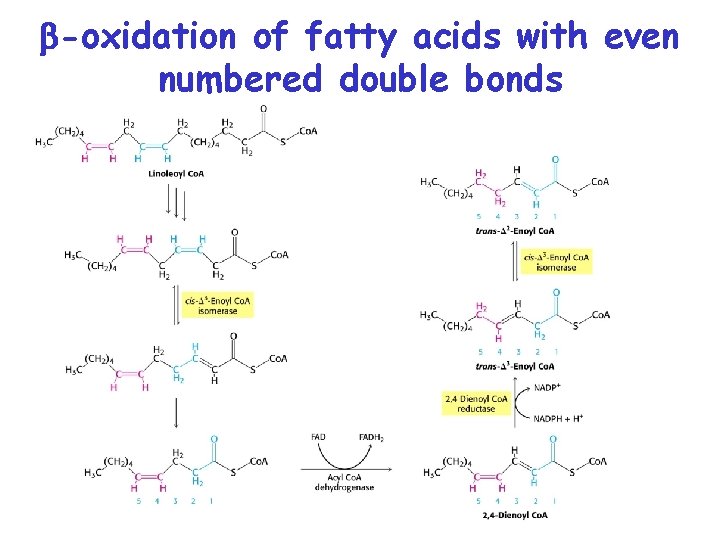
-oxidation of fatty acids with even numbered double bonds
Ketone Bodies • A special source of fuel and energy for certain tissues • Produced when acetyl-Co. A levels exceed the capacity of the TCA cycle (depends on OAA levels) • Under starvation conditions no carbos to produced anpleorotic intermediates • Some of the acetyl-Co. A produced by fatty acid oxidation in liver mitochondria is converted to acetone, acetoacetate and -hydroxybutyrate • These are called "ketone bodies" • Source of fuel for brain, heart and muscle • Major energy source for brain during starvation • They are transportable forms of fatty acids!
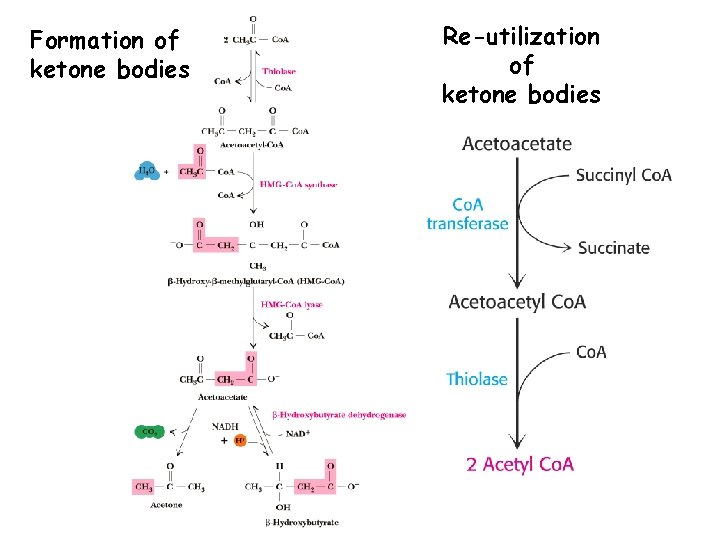
Formation of ketone bodies Re-utilization of ketone bodies

Ketone Bodies and Diabetes • Lack of insulin related to uncontrolled fat breakdown in adipose tissues • Excess b-oxidation of fatty acids results in ketone body formation. • Can often smell acetone on the breath of diabetics. • High levels of ketone bodies leads to condition known as diabetic ketoacidosis. • Because ketone bodies are acids, accumulation can lower blood p. H.
- Slides: 21