Fast and slow chemical reactions The speed at






- Slides: 9


Fast and slow chemical reactions The speed at which a chemical reaction occurs is known as the rate of reaction. Some chemical reactions that occur quickly are explosions and combustion reactions, such as the combustion of natural gas in a Bunsen burner and welding. These chemical reactions are said to have a fast rate of reaction. Chemical reactions that occur slowly are said to have a slow rate of reaction – for example rusting, ripening and the fermentation of grapes.

Controlling the rate of chemical reactions The rate of almost every reaction can be increased or decreased. For example, when you run a race you can start to breath harder and your heart pumps faster to speed up the rate of respiration. The rate of respiration slows down when you are calm and relaxed (example – animals slowing respiration in hibernation) Factors that affect rate of reaction: • Temperature • Concentration of the reactants • Surface area (chunks or powder) • Agitation (mixing or stirring) • Catalysts (chemical helpers) Change the variables – control the reactions!

Temperature Increasing temperature will normally increase the rate of chemical reaction. This occurs for two reasons: • It increases the speed of the particles in liquids and gases resulting in the particles colliding with each other more frequently so more chemical reactions in a shorter amount of time; • It gives the particles more energy. So when the molecules collide, they hit harder and the chemical bonds are more likely to breaking allowing the atoms and in the reactants to rearrange easily to form products.

Concentration The term concentration refers to the amount of a substance present in a certain volume of liquid or gas. For example 20 tea spoons of sugar has a higher concentration of sugar than one tea spoon. Increasing the concentration of reactants will increase the rate of reaction – meaning the particles are more likely to collide and therefore react when there are more of them.

Agitation Stirring reactants can also increase the rate of reaction. Stirring is known scientifically as agitation – and it ensures that the reactants are kept in contact. It does this by removing the build up of products around the reactants.

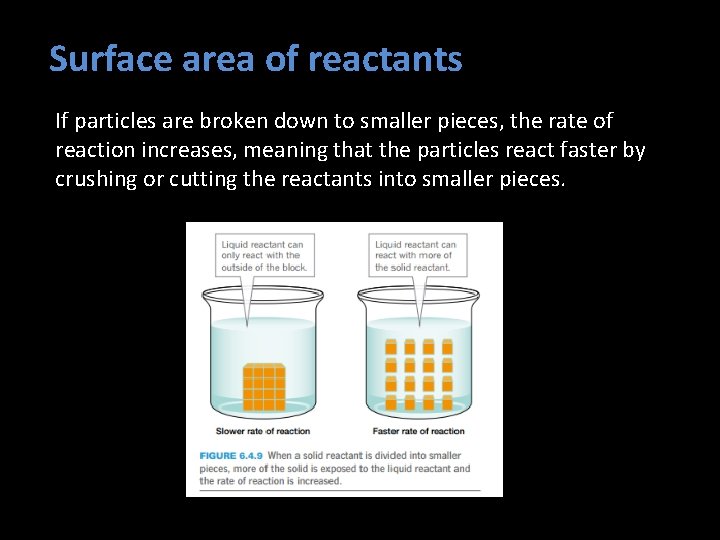
Surface area of reactants If particles are broken down to smaller pieces, the rate of reaction increases, meaning that the particles react faster by crushing or cutting the reactants into smaller pieces.

Catalysts are chemicals that speed yup chemical reactions but are not consumed (used up) during the reaction. They can be considered ‘chemical helpers’ that help[ the reactants to form the products. Catalysts can do this in two ways: • They reduce the amount of energy that is required to convert the reactants into products • They make it easier for reactant molecules to combine and form products. Cars use catalysts made of platinum in the exhaust systems – which is used to convert poisonous carbon monoxide into carbon dioxide

Enzymes are biological catalysts which means they naturally speed up chemical reactions in the body. They hold reactant molecules together until they rearrange to form products. This forces molecules to react that otherwise would not combine without the enzyme. Examples – the first step in digestion uses amylase found in saliva which breaks down starches in complex carbohydrates.