Farma Status Manual for Pharmaceutical companies Pharma Status









































- Slides: 68

Farma. Status Manual for Pharmaceutical companies

Pharma. Status Availability of medicines Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 2

1. About Pharma. Status 2. Section intended for the public 3. Section intended for pharmacists and wholesaler-distributors 4. Section intended for pharmaceutical companies 5. Exchange of information among various applications Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 3

1. About Pharma. Status § Content - Section intended for the public - Section intended for pharmacists and wholesaler-distributors - Section intended for pharmaceutical companies § URL § Version for mobile devices § General sections Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 4

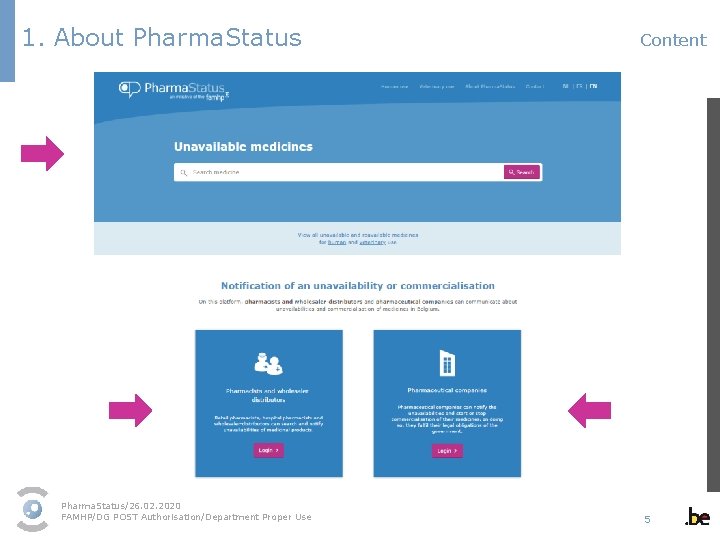
1. About Pharma. Status Content Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 5

1. About Pharma. Status URL Homepage EN version → https: //pharmastatus. be Homepage FR version → https: //pharmastatus. be Homepage NL version → https: //farmastatus. be Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 6

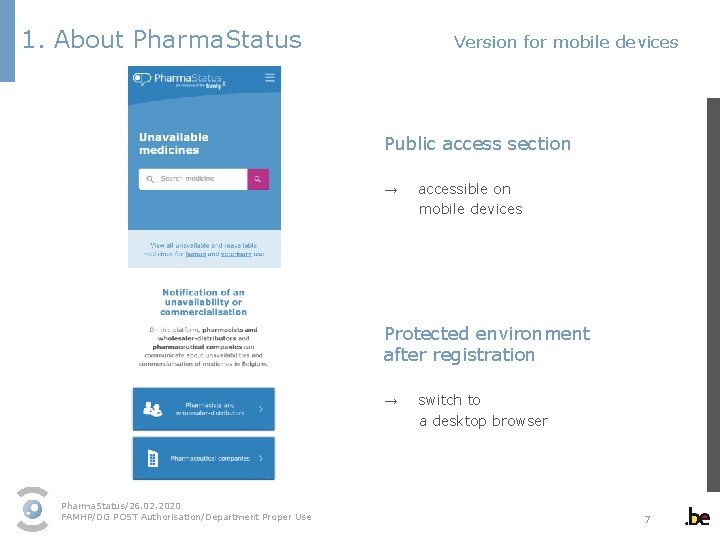
1. About Pharma. Status Version for mobile devices Public access section → accessible on mobile devices Protected environment after registration → switch to a desktop browser Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 7

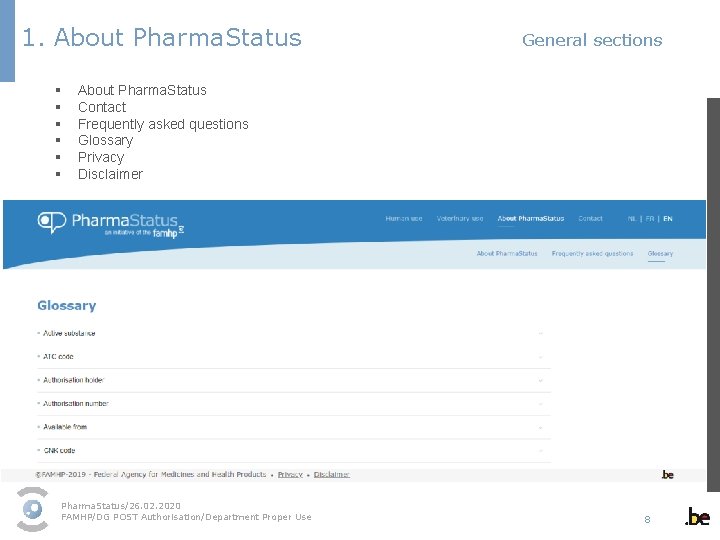
1. About Pharma. Status General sections § § § About Pharma. Status Contact Frequently asked questions Glossary Privacy Disclaimer Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 8

2. Public § Consultation of information on - unavailable medicines - unavailable and reavailable medicines for human use - unavailable and reavailable medicines for veterinary use § Registration to be kept informed on the unavailability of a medicine Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 9

2. Public Unavailable medicines General guidelines for publication Which unavailabilities are published? Temporary unavailability § with a presumed duration of the unavailability of at least 14 days § from 14 days before the notified start date of unavailability § published until the medicinal product is available again Interruption of commercialisation § as soon as the interruption of the commercialisation has been notified § published until the medicinal product is available again Stop of commercialisation § as soon as the stop of the commercialisation has been notified § published for 1 year after the stop of commercialisation Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 10

2. Public Unavailable and reavailable medicines Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 11

2. Public Reavailable medicines General guidelines for publication Which medicines available again are published? Medicines for which a temporary unavailability was notified, but which are now available again § any medicinal product for which there was a temporary unavailability published (in other words, with a presumed duration of the unavailability of at least 14 days) § as soon as the medicinal product is notified available again § published for 1 year after the medicinal product becomes available again Medicines for which an interruption of the commercialisation was notified, but which are now available again § as soon as the medicinal product is reported available again § published for 1 year after the medicinal product becomes available again Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 12

2. Public Reavailable medicines Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 13

2. Public Unavailable medicines Keep me informed Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 14

3. Pharmacists and wholesaler-distributors § Consultation of information on all unavailable and reavailable medicines § Contact the marketing authorisation holder or parallel distributor in the case of the unavailability of a medicinal product Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 15

4. Pharmaceutical companies § Send a notification to the FAMHP in case of a - temporary unavailability - interruption of commercialisation - stop of commercialisation - start of commercialisation § Answers to questions from pharmacist/wholesalerdistributors (next release) Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 16

4. Pharmaceutical companies § Registration as main user § Registration as additional user § Overview of my notifications § Search in all notifications § Send new notification § Modify notification § Cancel future notification § Recommendations for how to correctly send a notification Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 17

4. Pharmaceutical companies Registration as main user Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 18

4. Pharmaceutical companies Registration as main user At the release of Pharma. Status (once) Mail sent to generic e-mail address of all current marketing authorisation holders/parallel distributors ↓ After confirmation, this e-mail address is assigned as the main user and the company can log in to Pharma. Status In case of a new marketing authorisation holder/parallel distributor Generic e-mail address is completed in the FAMHP database for the relevant marketing authorisation holder/parallel distributor (if known) ↓ Mail sent to generic e-mail address of the company ↓ After confirmation, this e-mail address is assigned as the main user and the marketing authorisation holder/parallel distributor can log in to Pharma. Status Request a login via the website Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 19

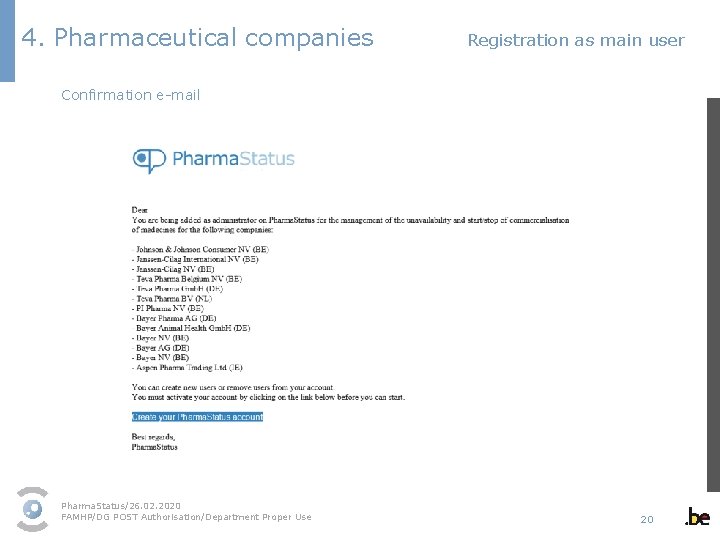
4. Pharmaceutical companies Registration as main user Confirmation e-mail Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 20

4. Pharmaceutical companies Registration as main user Edit profile Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 21

4. Pharmaceutical companies Registration as main user What if you want to change the e-mail address of the main user? Send an e-mail to database@fagg. be ↓ Previous e-mail address is deleted for the relevant company. New e-mail address is added for the relevant company. ↓ You will receive a confirmation e-mail. After confirmation, you can log in with the new e-mail address. Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 22

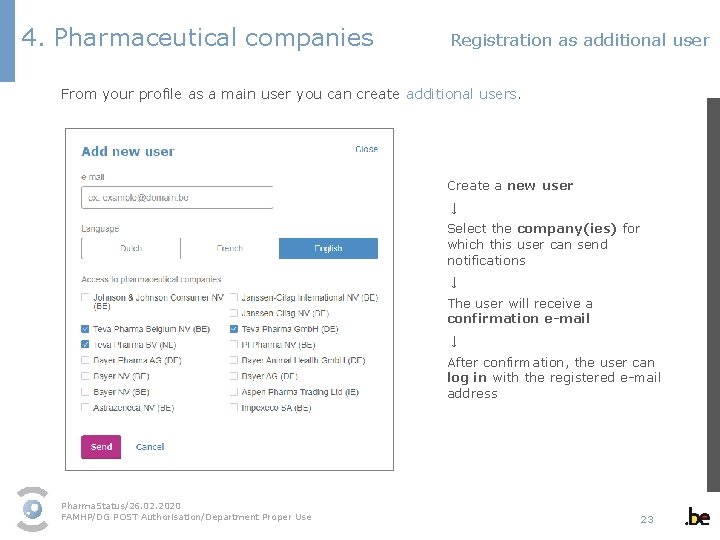
4. Pharmaceutical companies Registration as additional user From your profile as a main user you can create additional users. Create a new user ↓ Select the company(ies) for which this user can send notifications ↓ The user will receive a confirmation e-mail ↓ After confirmation, the user can log in with the registered e-mail address Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 23

4. Pharmaceutical companies Registration as additional user You can remove a user of edit the profile at any time. Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 24

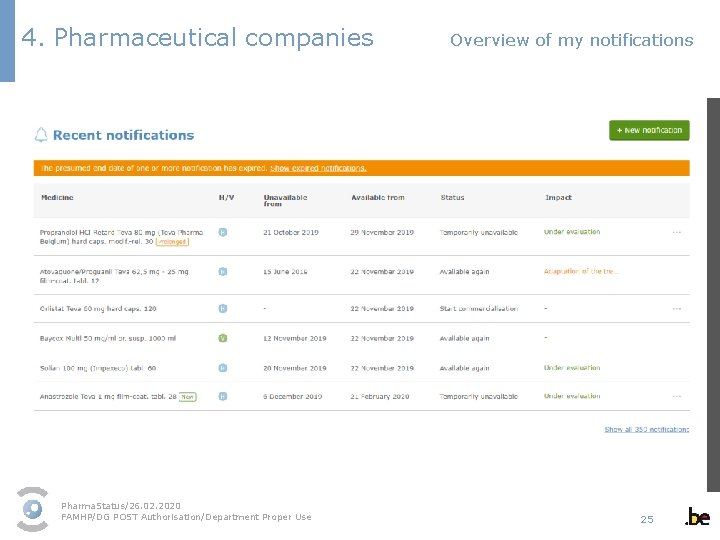
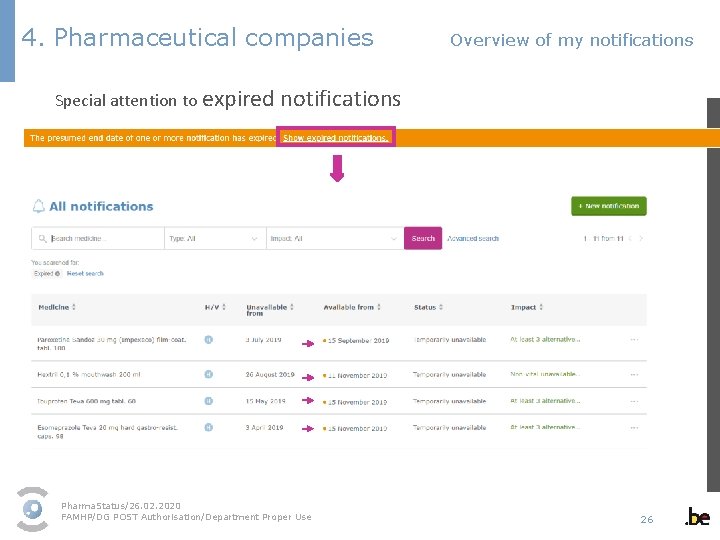
4. Pharmaceutical companies Overview of my notifications Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 25

4. Pharmaceutical companies Overview of my notifications Special attention to expired notifications Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 26

4. Pharmaceutical companies Overview of my notifications Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 27

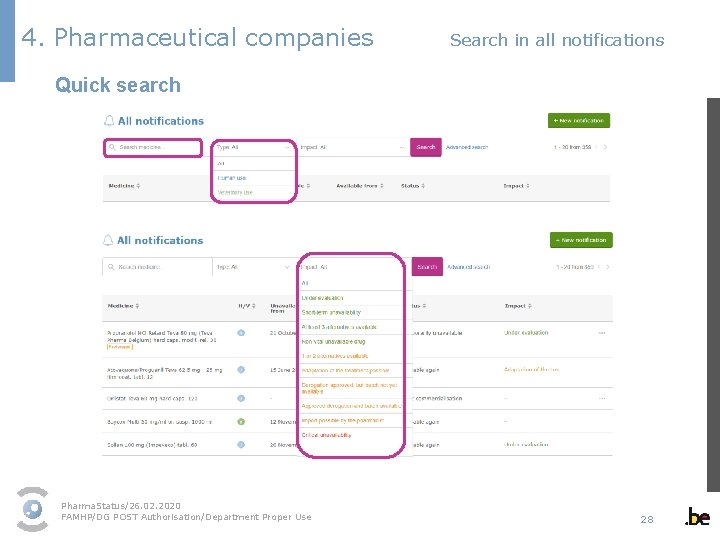
4. Pharmaceutical companies Search in all notifications Quick search Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 28

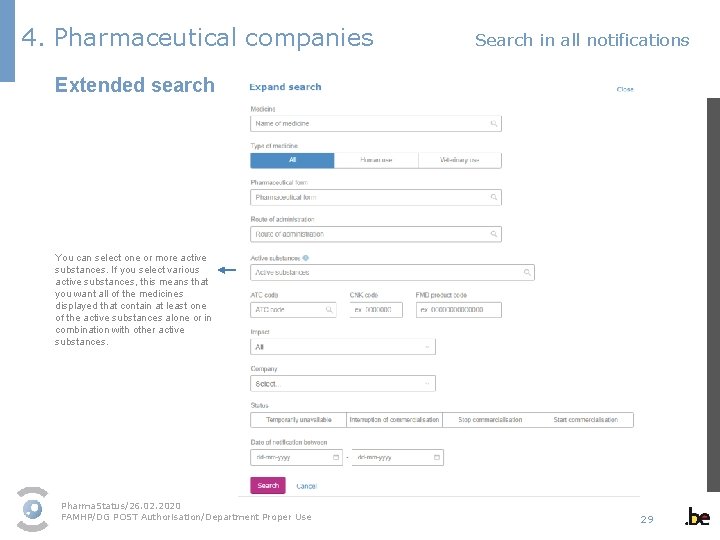
4. Pharmaceutical companies Search in all notifications Extended search You can select one or more active substances. If you select various active substances, this means that you want all of the medicines displayed that contain at least one of the active substances alone or in combination with other active substances. Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 29

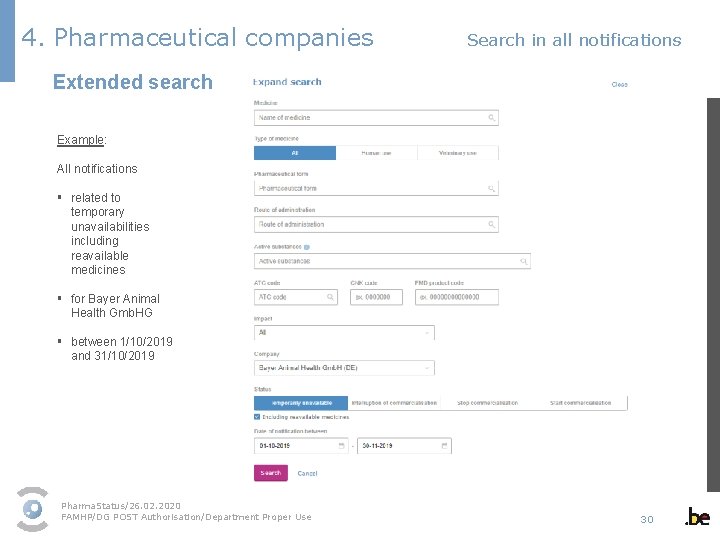
4. Pharmaceutical companies Search in all notifications Extended search Example: All notifications § related to temporary unavailabilities including reavailable medicines § for Bayer Animal Health Gmb. HG § between 1/10/2019 and 31/10/2019 Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 30

4. Pharmaceutical companies Search in all notifications Search results Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 31

4. Pharmaceutical companies Search in all notifications Search results ↓ Download – Medicinal product – Status – Unavailable from – Unavailable until (presumed end date) – Available from – Reason – Impact – Additional information – Marketing authorisation holder/parallel distributor – Marketing authorisation number – CTI Extended – CNK code – FMD product code – ATC code – Reported on Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 32

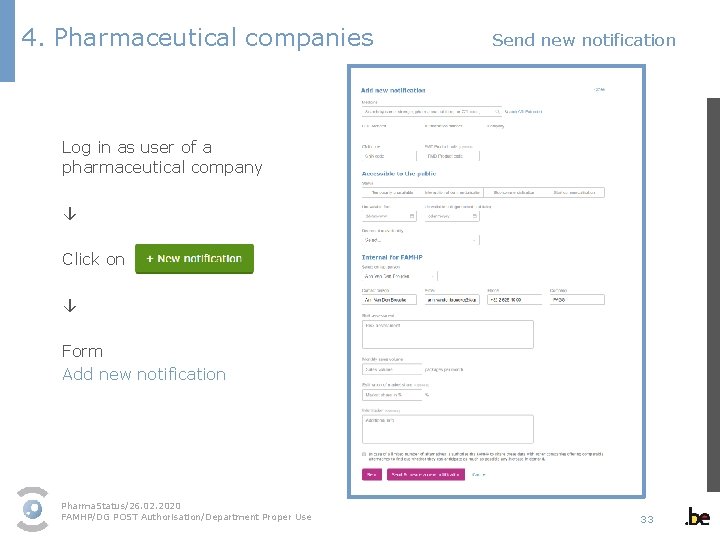
4. Pharmaceutical companies Send new notification Log in as user of a pharmaceutical company ↓ Click on ↓ Form Add new notification Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 33

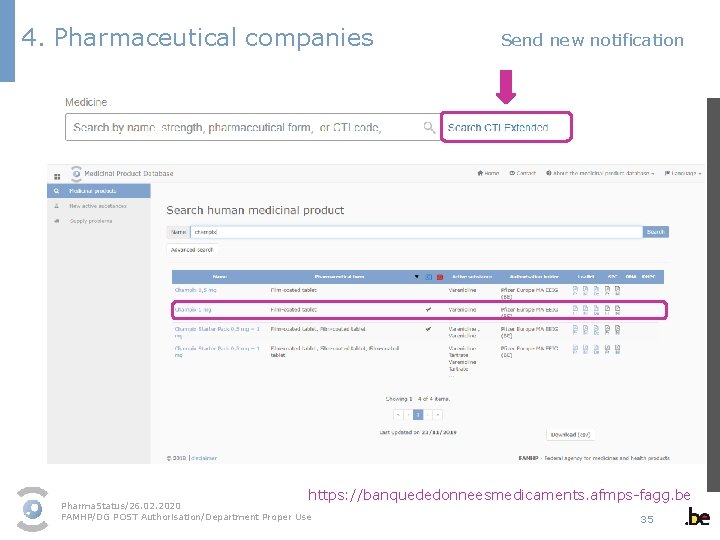
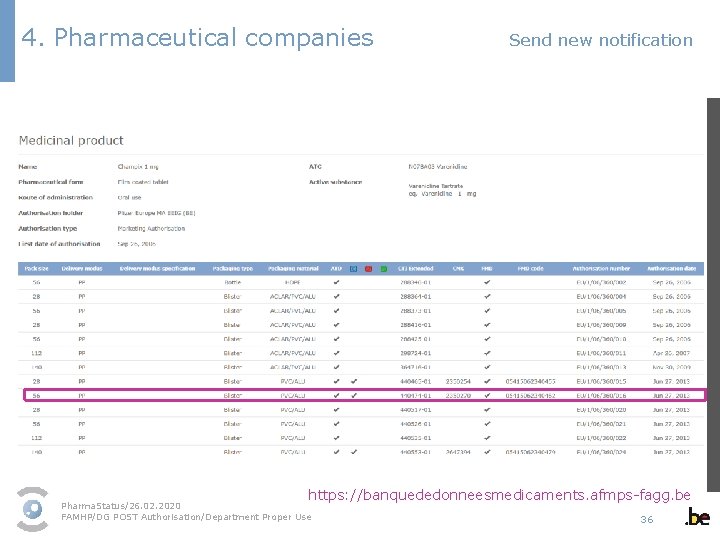
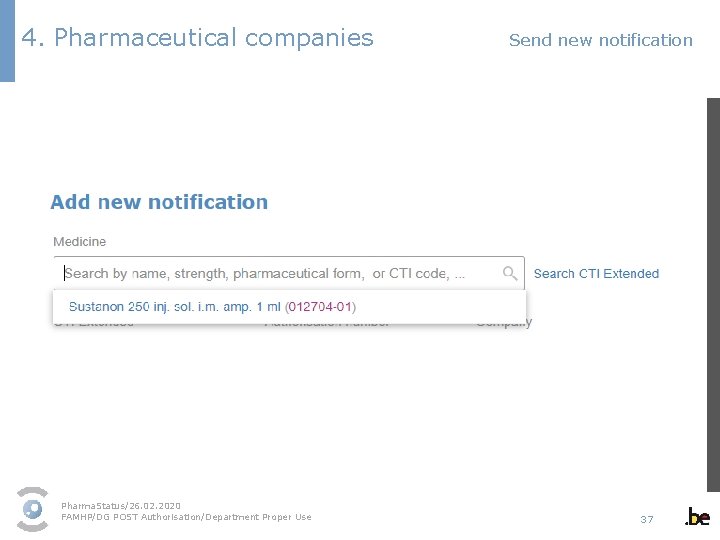
4. Pharmaceutical companies Send new notification 1 - Select the medicinal product packaging in the dropdown menu § As a user, you can only select the medicines of the marketing authorisation holders/parallel distributors listed in your profile. § The dropdown menu contains all authorised packagings. Pack sizes/medicines that have been revoked or suspended cannot be commercialised and as a result are not displayed. § Select the correct packaging. If you are not sure, you can find an overview of all authorised packagings in the medicinal product database available on the FAMHP website via the link “Search CTI Extended”. https: //banquededonneesmedicaments. afmps-fagg. be Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 34

4. Pharmaceutical companies Send new notification https: //banquededonneesmedicaments. afmps-fagg. be Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 35

4. Pharmaceutical companies Send new notification https: //banquededonneesmedicaments. afmps-fagg. be Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 36

4. Pharmaceutical companies Send new notification Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 37

4. Pharmaceutical companies Send new notification 2 - Medicinal product characteristics CTI Extended cannot be changed, unique ID originating from the FAMHP database Marketing authorisation number cannot be changed → ask for corrections via database@fagg. be Company cannot be changed → ask for corrections via database@fagg. be CNK code can be filled in/edited via the form - required field for medicines for human use - public CNK code → validation rules of APB are followed (see next slide) FMD product code can be filled in/edited via the form - required field for medicines subject to FMD regulation - 14 characters Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 38

4. Pharmaceutical companies Send new notification Validation rules public CNK codes that fall within the following ranges are not allowed as public CNK code Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 39

4. Pharmaceutical companies Send new notification 3 - Publicly accessible information = data shared with third parties, including via the public access section of Pharma. Status, website of the FAMHP, . . . Status Depending on the data available in the FAMHP database for the selected pack, one of the following statuses can be selected: → Temporarily unavailable → Interruption of commercialisation → Stop of commercialisation → Start of commercialisation Reason Choice of a value from a dropdown If the reason is not consistent with one of these values → option of selecting “Other reason” + specify in the field Information (see further) Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 40

4. Pharmaceutical companies Send new notification Status Temporarily unavailable = temporary unavailability of a medicine for a short duration (maximum 1 year) § Unavailable from § Unavailable until § Reason = = = start date of the temporary unavailability presumed end date of the temporary unavailability (max. 1 year after the start date) reason for temporary unavailability Interruption of commercialisation = commercialisation is stopped for a longer period of time (usually longer than one year), but the intention is that the medicine will be commercialised again § Unavailable from § Reason = = start date of interruption of commercialisation reason for interruption of commercialisation Stop of commercialisation = commercialisation is definitively stopped § Unavailable from § Reason = = start date of stop of commercialisation reason for stop of commercialisation Start of commercialisation § Available from = start date of commercialisation Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 41

4. Pharmaceutical companies Send new notification Status that can be selected depends on the current status of the selected pack. Medicine A is not commercialised Medicine B is commercialised Medicine C is temporarily unavailable Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 42

4. Pharmaceutical companies Send new notification 4 - Intern for the FAMHP = data that are not published, but used for the evaluation of the impact of the unavailability. Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 43

4. Pharmaceutical companies Send new notification Contact After logging in, your personal information will be loaded by default. You can change these data. In case of an unavailability, the following information may be useful in the evaluation of the impact. Risk assessment (Required field) Analysis by the company of the severity of the unavailability of the medicinal product including: § alternatives, § solutions, § residual stock (possibly also abroad), § … Monthly sales volume (Required field) Average number of packs sold per month under normal conditions (not taking into account a possible increase as a result of the unavailability of another medicine) Estimated market share Market share of the concerned medicine This is important information in the evaluation of the impact of the unavailability. Information Additional information about the unavailability Example: clarification of the reason if choosing “Other reason” Permission to communicate the monthly sales volume to other companies, for the unavailability of essential medicines with a limited number of equivalent alternatives and exclusively with the intention of finding a solution for the unavailability. Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 44

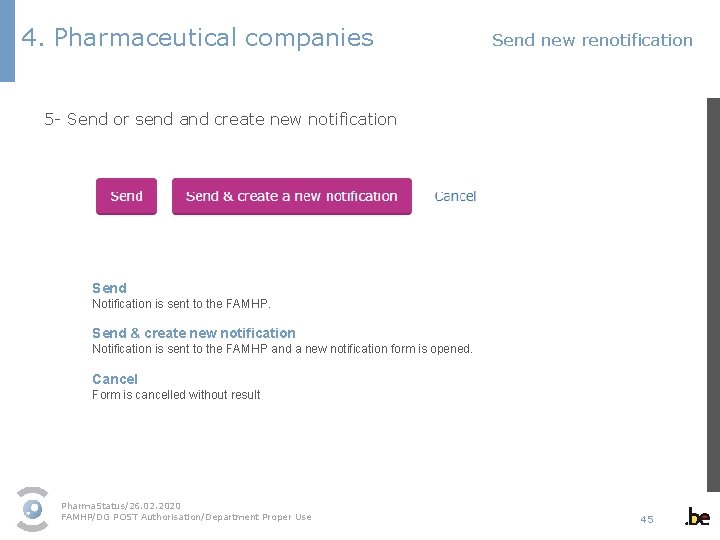
4. Pharmaceutical companies Send new renotification 5 - Send or send and create new notification Send Notification is sent to the FAMHP. Send & create new notification Notification is sent to the FAMHP and a new notification form is opened. Cancel Form is cancelled without result Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 45

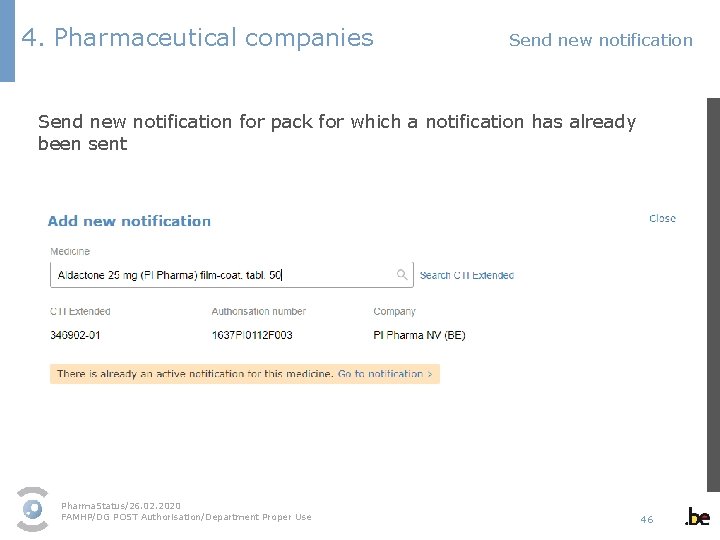
4. Pharmaceutical companies Send new notification for pack for which a notification has already been sent Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 46

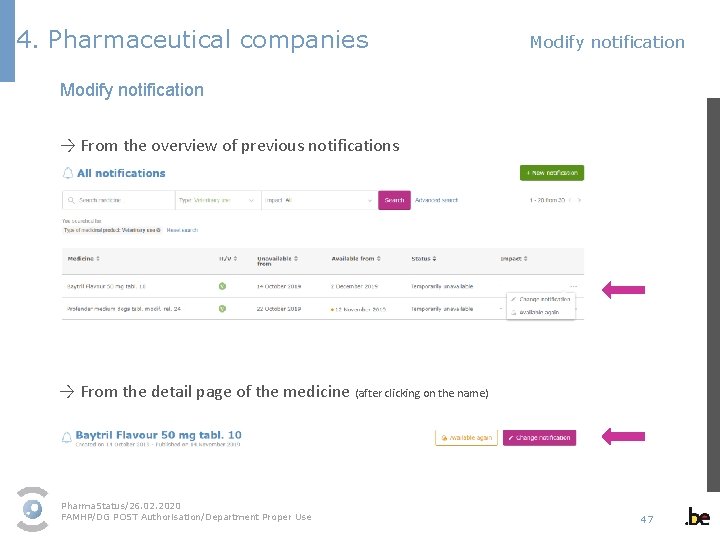
4. Pharmaceutical companies Modify notification → From the overview of previous notifications → From the detail page of the medicine (after clicking on the name) Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 47

4. Pharmaceutical companies Modify notification Status that can be selected depends on the current status of the selected pack. Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 48

4. Pharmaceutical companies Modify notification Good to know When changing the notification or notifying the end of a temporary unavailability ↓ Those who have registered via automatically receive an e-mail with an update regarding the unavailability of the medicine. Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 49

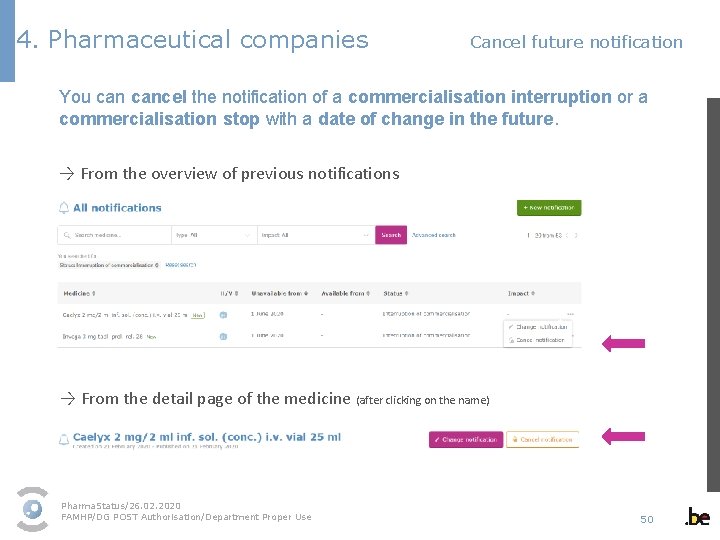
4. Pharmaceutical companies Cancel future notification You cancel the notification of a commercialisation interruption or a commercialisation stop with a date of change in the future. → From the overview of previous notifications → From the detail page of the medicine (after clicking on the name) Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 50

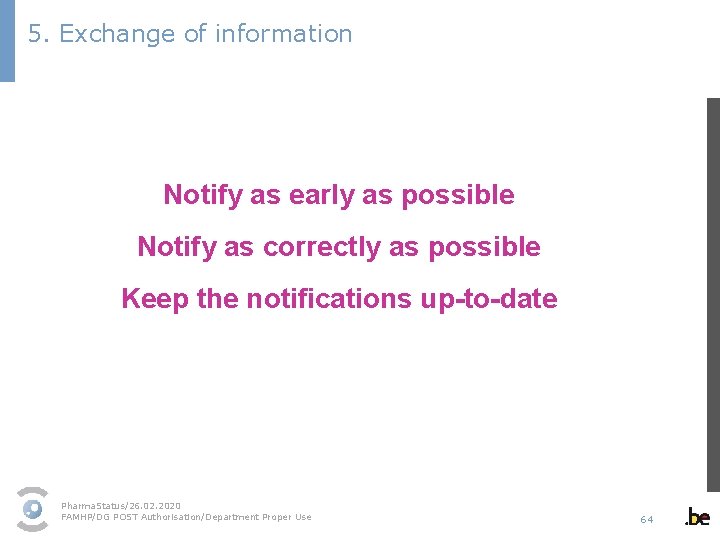
4. Pharmaceutical companies Recommendations When do I send a report? Notify as early as possible Notify as correctly as possible Keep the notifications up-to-date ! Important for § correct evaluation of the impact 1. Short term unavailability Þ few problems expected Þ no further action taken 2. Only if prolongation of the unavailability, thorough evaluation => much valuable time lost § correct informing of patients § correct informing of pharmacists → After registration: e-mail is sent after each update => Efficient notification ensures efficient information § correct informing of doctors → Anticipation when prescribing a medicine Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 51

4. Pharmaceutical companies Recommendations When do I send a report? Start of commercialisation § as soon as the effective commercialisation date is known § at least fifteen working days before the effective commercialisation date Stop of commercialisation § at least six months before the effective stop date Interruption of commercialisation § as soon as the date of the interruption of commercialisation is known § at the latest 7 days after the start of the interruption Temporary unavailability § in case of a unavailability expected to last at least 14 days § as soon as the date of the unavailability is known § at the latest 7 days after the start of the unavailability § also notify partial unavailabilities, not just complete out of stocks § reliable presumed end date Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 52

4. Pharmaceutical companies Recommendations What if commercialisation is delayed? Notification “start commercialisation” is sent ↓ Commercialisation is delayed ↓ Modify start date in previously sent notification ! Do not report as a temporary unavailability A temporary unavailability is not applicable to a medicine that was never available on the Belgian market. ! Important to send a delay of commercialisation before the previously notified start date of the commercialisation → impact on prescription software Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 53

4. Pharmaceutical companies Recommendations What happens if a medicine is revoked? Revocation of the marketing authorisation in the FAMHP database ↓ Medicine with commercialisation status = yes Automatic update of data in the FAMHP database Þ commercialisation is stopped Þ date of stop of commercialisation = date of revocation of the marketing authorisation Þ reason for stop of commercialisation = « revocation of marketing autorisation » Medicine with commercialisation status = yes and temporary unavailability Automatic update of data in the FAMHP database Þ Þ Þ commercialisation is stopped date of end of commercialisation = date of revocation of the marketing authorisation reason for end of commercialisation = « revocation of marketing autorisation » temporary unavailability is closed date of end of temporary unavailability = date of revocation of marketing authorisation Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 54

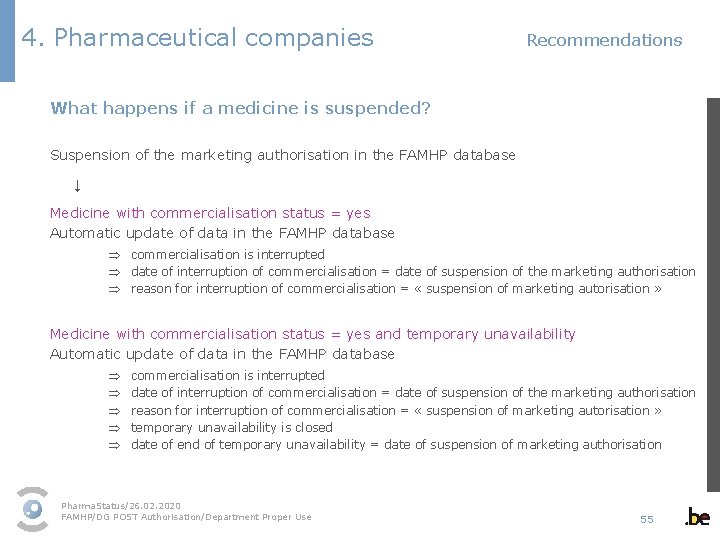
4. Pharmaceutical companies Recommendations What happens if a medicine is suspended? Suspension of the marketing authorisation in the FAMHP database ↓ Medicine with commercialisation status = yes Automatic update of data in the FAMHP database Þ commercialisation is interrupted Þ date of interruption of commercialisation = date of suspension of the marketing authorisation Þ reason for interruption of commercialisation = « suspension of marketing autorisation » Medicine with commercialisation status = yes and temporary unavailability Automatic update of data in the FAMHP database Þ Þ Þ commercialisation is interrupted date of interruption of commercialisation = date of suspension of the marketing authorisation reason for interruption of commercialisation = « suspension of marketing autorisation » temporary unavailability is closed date of end of temporary unavailability = date of suspension of marketing authorisation Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 55

4. Pharmaceutical companies Recommendations What happens if a medicine is suspended? Important If the medicine is again commercialised after the lifting of a suspension, a “start of commercialisation” notification must be sent. The previous status is not automatically resumed in the database. Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 56

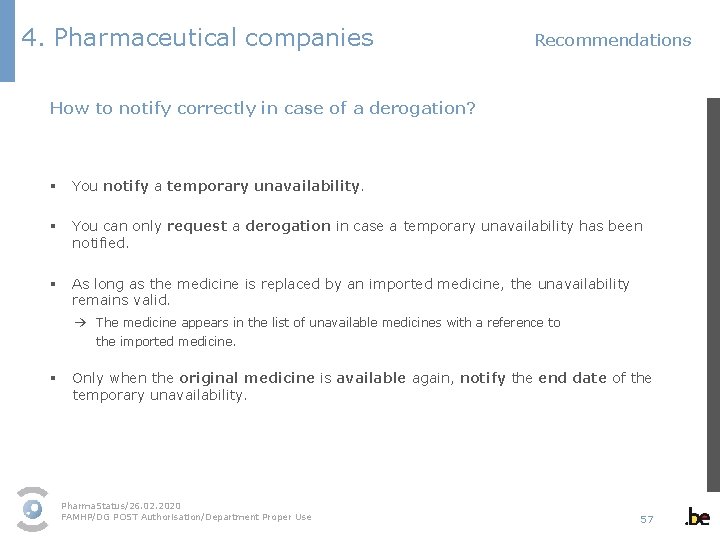
4. Pharmaceutical companies Recommendations How to notify correctly in case of a derogation? § You notify a temporary unavailability. § You can only request a derogation in case a temporary unavailability has been notified. § As long as the medicine is replaced by an imported medicine, the unavailability remains valid. → The medicine appears in the list of unavailable medicines with a reference to the imported medicine. § Only when the original medicine is available again, notify the end date of the temporary unavailability. Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 57

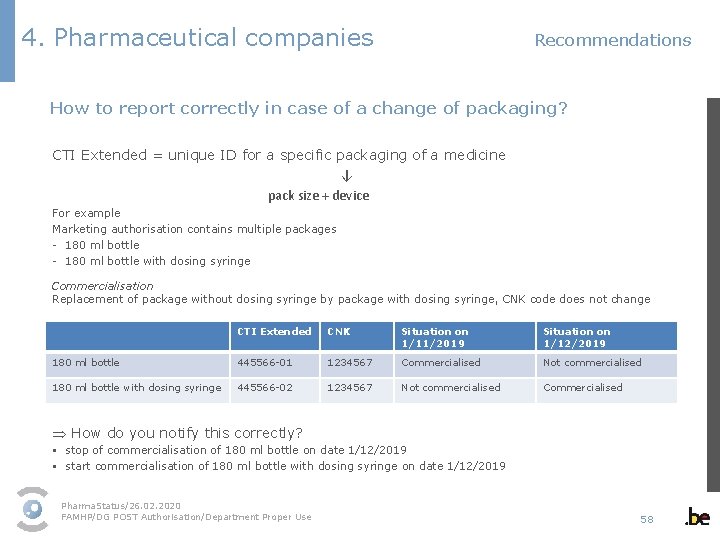
4. Pharmaceutical companies Recommendations How to report correctly in case of a change of packaging? CTI Extended = unique ID for a specific packaging of a medicine ↓ pack size + device For example Marketing authorisation contains multiple packages - 180 ml bottle with dosing syringe Commercialisation Replacement of package without dosing syringe by package with dosing syringe, CNK code does not change CTI Extended CNK Situation on 1/11/2019 Situation on 1/12/2019 180 ml bottle 445566 -01 1234567 Commercialised Not commercialised 180 ml bottle with dosing syringe 445566 -02 1234567 Not commercialised Commercialised Þ How do you notify this correctly? § stop of commercialisation of 180 ml bottle on date 1/12/2019 § start commercialisation of 180 ml bottle with dosing syringe on date 1/12/2019 Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 58

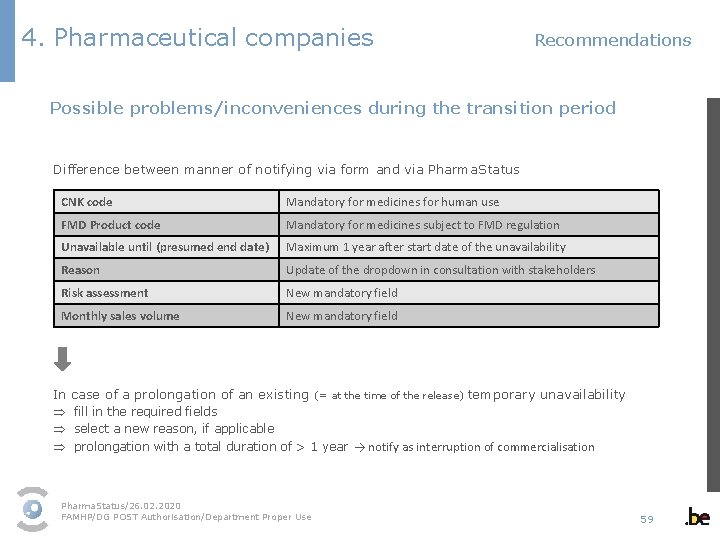
4. Pharmaceutical companies Recommendations Possible problems/inconveniences during the transition period Difference between manner of notifying via form and via Pharma. Status CNK code Mandatory for medicines for human use FMD Product code Mandatory for medicines subject to FMD regulation Unavailable until (presumed end date) Maximum 1 year after start date of the unavailability Reason Update of the dropdown in consultation with stakeholders Risk assessment New mandatory field Monthly sales volume New mandatory field In case of a prolongation of an existing (= at the time of the release) temporary unavailability Þ fill in the required fields Þ select a new reason, if applicable Þ prolongation with a total duration of > 1 year → notify as interruption of commercialisation Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 59

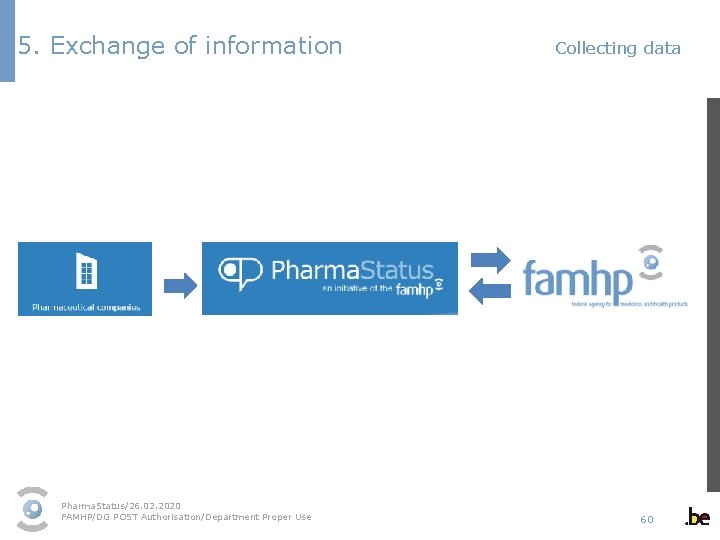
5. Exchange of information Collecting data Patients Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 60

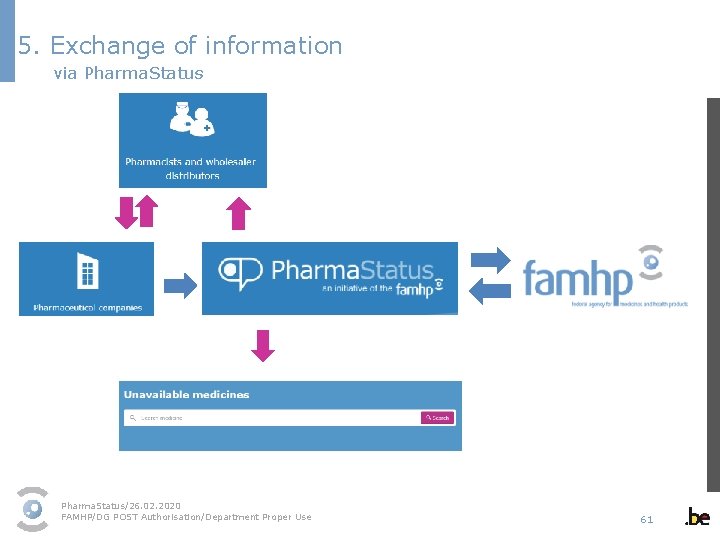
5. Exchange of information via Pharma. Status Patients Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 61

5. Exchange of information via other channels … SAM Authentic Medicines Database Patients Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 62

5. Exchange of information Authentic national medicines database SAMv 2 from 1/1/2020 obligatory use of SAMv 2 as source of information for electronic prescriptions Recommendations for correct use of SAMv 2 when including medicine data in the prescription software of doctors → included in the homologation conditions of the software applications (1/10/2020) If applicable and available in the SAMv 2 database, the following information needs to be visible to the prescriber at the moment he selects a medicinal product. The information has to be shown in a clear, proactive way next to the concerned pack size (e. g. in the form of a sign indicating additional information via a hovering action). Temporary supply Problem = symbol indicating there is an actual temporary supply problem of the concerned pack size of the medicinal product. Additional information (e. g. via hovering) contains the following data: §start date supply problem, §presumed end date supply problem, §reason supply problem, §impact supply problem, §additional information concerning alternative medicinal products or treatments. End of commercialisation = symbol indicating the end of commercialisation of the concerned pack size of the medicinal product. Additional information (e. g. via hovering) contains the following data: §reason end of commercialisation, §impact end of commercialisation, §additional information concerning alternative medicinal products or treatments. Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use http: //www. samportal. be 63

5. Exchange of information Notify as early as possible Notify as correctly as possible Keep the notifications up-to-date Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 64

5. Exchange of information Carly Fiorina, former executive, president, and chair of Hewlett-Packard Co. Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 65

If you notice an error or have a comment about information notified in Pharma. Status, contact database@fagg. be. If you have another question about the availability of a medicine, contact supply-problems@fagg. be. Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 66

Contact Federal Agency for Medicines and Health Products FAMHP Victor Hortaplein 40/40 1060 BRUSSELS Tel. : + 32 2 528 40 00 fax + 32 2 528 40 01 e-mail welcome@fagg. be www. fagg. be Follow the FAMHP on Facebook, Twitter and Linked. In Pharma. Status/26. 02. 2020 FAMHP/DG POST Authorisation/Department Proper Use 67

Your medicines and health products, our care
 Pharma status
Pharma status Pharmaceutical companies in georgia
Pharmaceutical companies in georgia Good publication practice for pharmaceutical companies
Good publication practice for pharmaceutical companies Farma zvířat rozbor
Farma zvířat rozbor Kalbe farma
Kalbe farma životinjska farma lektira
životinjska farma lektira Kasus manajemen laba
Kasus manajemen laba Farma delivery jp
Farma delivery jp Koskipalvelu
Koskipalvelu Keluarga jahja santoso
Keluarga jahja santoso Objekti za tov junadi
Objekti za tov junadi Farma němetice
Farma němetice životinjska farma 7 zapovijedi
životinjska farma 7 zapovijedi Smk farmasi madiun
Smk farmasi madiun Kung dog 1611
Kung dog 1611 Densitet vatten
Densitet vatten Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Tack för att ni har lyssnat
Tack för att ni har lyssnat Smärtskolan kunskap för livet
Smärtskolan kunskap för livet Mall för referat
Mall för referat Karttecken kraftledning
Karttecken kraftledning Mjälthilus
Mjälthilus Vätsketryck formel
Vätsketryck formel Samlade siffror för tryck
Samlade siffror för tryck Delegerande ledarstil
Delegerande ledarstil Toppslätskivling dos
Toppslätskivling dos Elektronik för barn
Elektronik för barn Adressändring ideell förening
Adressändring ideell förening Borra hål för knoppar
Borra hål för knoppar Bris för vuxna
Bris för vuxna Bra mat för unga idrottare
Bra mat för unga idrottare Etik och ledarskap etisk kod för chefer
Etik och ledarskap etisk kod för chefer Trög för kemist
Trög för kemist Teckenspråk minoritetsspråk argument
Teckenspråk minoritetsspråk argument Humanitr
Humanitr Datorkunskap för nybörjare
Datorkunskap för nybörjare Returpilarna
Returpilarna Steg för steg rita
Steg för steg rita Redogör för vad psykologi är
Redogör för vad psykologi är Geometriska former i förskolan
Geometriska former i förskolan Claes martinsson
Claes martinsson Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Svenskt ramverk för digital samverkan
Svenskt ramverk för digital samverkan Vad är lyrik
Vad är lyrik Tidböcker
Tidböcker Datumr
Datumr Klädsel i rom
Klädsel i rom Orubbliga rättigheter
Orubbliga rättigheter Verktyg för automatisering av utbetalningar
Verktyg för automatisering av utbetalningar Ministerstyre för och nackdelar
Ministerstyre för och nackdelar Jätte råtta
Jätte råtta Plats för toran ark
Plats för toran ark Exspektans eller expektans
Exspektans eller expektans Tillitsbaserad ledning
Tillitsbaserad ledning Ro i rom pax
Ro i rom pax Stig kerman
Stig kerman Varför kallas perioden 1918-1939 för mellankrigstiden
Varför kallas perioden 1918-1939 för mellankrigstiden Informationskartläggning
Informationskartläggning Borstål, egenskaper
Borstål, egenskaper Vishnuismen
Vishnuismen Centrum för kunskap och säkerhet
Centrum för kunskap och säkerhet Påbyggnader för flakfordon
Påbyggnader för flakfordon Lyckans minut erik lindorm analys
Lyckans minut erik lindorm analys Inköpsprocessen steg för steg
Inköpsprocessen steg för steg Anatomi organ reproduksi
Anatomi organ reproduksi Strategi för svensk viltförvaltning
Strategi för svensk viltförvaltning Typiska drag för en novell
Typiska drag för en novell Stickprovsvariansen
Stickprovsvariansen Rutin för avvikelsehantering
Rutin för avvikelsehantering