FAOWHO CODEX ALIMENTARIUS COMMISSION CODEX Joint FAOWHO Food
















































- Slides: 56

FAO/WHO CODEX ALIMENTARIUS COMMISSION (CODEX) Joint FAO/WHO Food Standards Programme Food and Agriculture Organization of the United Nations Lusaka, Zambia, 3 -5 July 2002

1. Introducing Codex Alimentarius Commission 2. Role of Codex in setting food standards and the SPS Agreement Implementation 3. Import/Export food control Why rejections? 4. Conclusions

Codex Alimentarius Latin for “Food Law” or “Food Code”

What is “Codex” n The Codex Alimentarius Commission u. Committees and Task Forces u. Secretariat n The Codex Alimentarius u. Standards and residue limits u. Code of practices, guidelines and Recommendations (13 Volumes) n The Codex “Process”

Codex Alimentarius Commission n n Founded by FAO in 1961 Responsible for the Joint FAO/WHO Food Standards Programme since 1962

Codex Objectives n n n To protect the health of consumers To ensure fair practices in the food trade To coordinate all food standards work

Codex Alimentarius Commission n n 167 Member countries u. Observers from IGOs & INGOs Establishes its own programme of work Adopts standards, guidelines and other recommendations Makes recommendations to Member governments, FAO and WHO on general food standards matters

The Commission n Meets every 2 years u. Rome n n or Geneva Meetings last for 6 days Works in Arabic*, Chinese*, English, French and Spanish *(From 2001)

Codex Alimentarius Commission n n 1 st Session of the Codex Alimentarius Commission, Rome, October 1963 u 120 participants from 30 countries and 16 international organizations 24 Session of the Codex Alimentarius Commission, Geneva, June-July 2001 u 608 participants from 104 countries and 63 international organizations

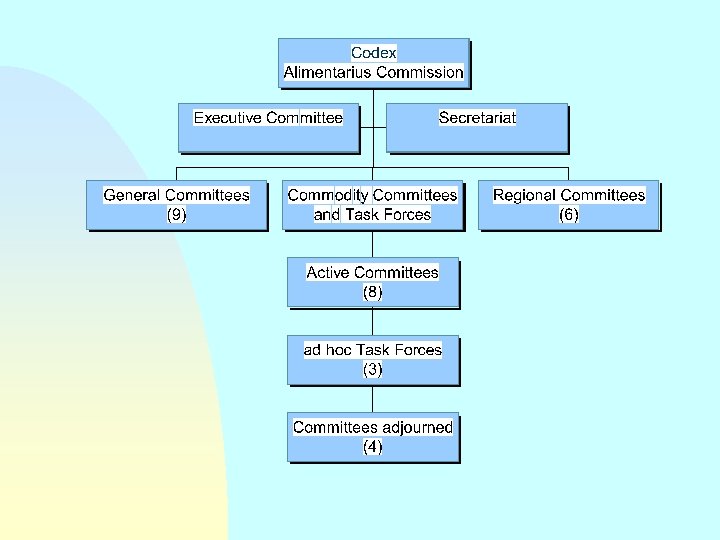
STRUCTURE OF THE CODEX ALIMENTARIUS COMMISSION


Management Organs of the Commission n The Executive Committee The Regional Coordinating Committees The Secretariat of the Commission

The Executive Committee n Chairperson n 3 Vice Chairpersons n n 7 Regional Representatives (governments) 6 Regional Coordinators (observers)

The Regional Coordinating Committees n 6 Regional Committees Africa, Asia, Europe, Near East, Latin America & Caribbean, North America & Southwest Pacific n Coordinate activities relevant to the region u. Regional Codex u. Harmonization standards

The Secretariat of the Commission Administrative support to the Commission n Link with Codex Contact Points n Co-ordination with the work of other organs n Located at HQ of FAO (Rome) n

The Secretariat Acts as the link with Codex Contact Points of Member countries

Technical Organs of the Commission 9 General Subject Committees + 12 Commodity Committees + 3 Ad Hoc Inter-Governmental Task Forces

General Subject Committees n n General Principles (France) n Food Additives & Contaminants (Netherlands) n Food Labelling (Canada) n Food Hygiene (USA) n Pesticide Residues n (Netherlands) n Methods of Analysis & Sampling (Hungary) n Food Import and Export Inspection and Certification Systems (Australia) Residues of Veterinary Drugs in Foods (USA) Nutrition & Foods for Special Dietary Uses (Germany)

Codex Commodity Committees n Processed Fruits and Vegetables n (USA) n n n Fresh Fruits and Vegetables (Mexico) Milk and Milk Products (New Zealand) Fats and Oils (UK) n n Fish and Fishery Products (Norway) Natural Mineral Waters (Switzerland) Cocoa Products and Chocolate (Switzerland) n Sugars (UK)

Codex Committees adjourned n Mineral Waters n Cereals, Pulses and Legumes n Vegetable Proteins n Soups and Broths

Ad Hoc Intergovernmental Codex Task Forces (established by the 23 rd Session of the CAC) n n n Foods derived from Biotechnology (Japan, 14 -17 March 2000) Animal Feeding (Denmark, 13 -15 June 2000) Fruit and Vegetable Juices (Brazil, 18 -22 September 2000)

Expert input to Codex Committees Joint FAO/WHO Expert Committee on Food Additives (JECFA) Joint FAO/WHO Meeting on Pesticide Residues (JMPR) Joint FAO/WHO Expert Consultations

ELABORATION PROCEDURES

1 M R O F I E N R U U D E C PRO TEPS) (8 S Possible Omission Decision of the Commission 2 Proposed draft standard 3 Request of written Comments 4 Amendments / Session 5 Adoption as a draft standard 6 Request of written Comments 7 Amendments / Session 8 Adoption as a Codex standard

d e at c c A r e el 1 Decision of the Commission (vote) 2 Proposed draft standard 3 Request of written Comments 4 Amendments / Session 5 Adoption as a Codex Standard

Achievements 237 Food Standards 43 Codes of Practice 33 Guidelines 197 Pesticides evaluated 3274 Limits for pesticides residues 25 Guideline limits for contaminants 54 Veterinary drugs evaluated 289 Limits of veterinary drug residues 1300 Food additives evaluated

Codex and the SPS Agreement

SPS AGREEMENT Important Elements n Basic Rights n Risk Assessment n Harmonization n Equivalence n Transparency n Technical Assistance

SPS Agreement Discourages the use of SPS measures as barriers to international trade n Recognizes Codex as a reference on food safety n Codex may be used to settle disputes n Calls for harmonization based on Codex n

Implications of SPS on Codex focuses on risk-based inspection and certification systems u. Inclusion of HACCP in the General Principles of Food Hygiene u. Development of import/export food inspection and certification guidelines u. Work on Risk Analysis - Risk Assessment n n Codex reaffirms the role of science in its work Codex revised its acceptance rules

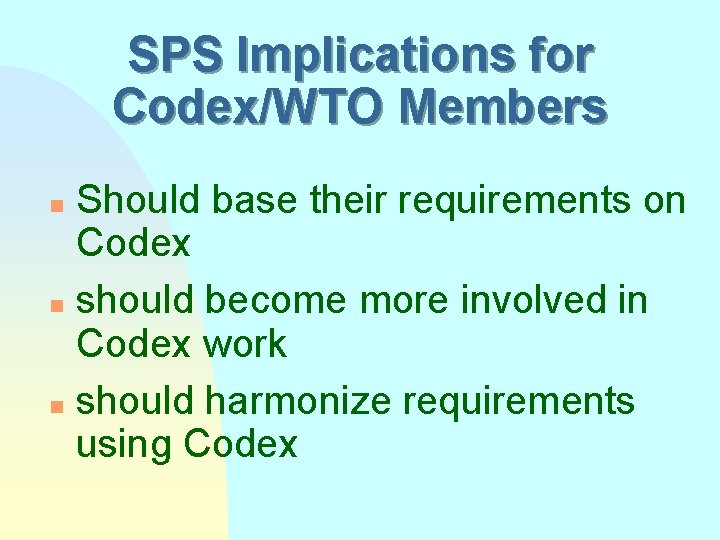
SPS Implications for Codex/WTO Members Should base their requirements on Codex n should become more involved in Codex work n should harmonize requirements using Codex n

Who is responsible for what? Government Industry Consumer

Government’s responsibility ü ü Protect the public health Ensure fair practices in the food trade

How does government meet its responsibility? ü establishment of appropriate legislation and regulations ü communication of food quality and safety requirements to industry ü provision of training to industry to ensure their ability to develop and implement adequate quality assurance programmes

What is industry’s responsibility? To produce safe food of acceptable quality How does it meet its responsibility? By establishing and maintaining adequate quality assurance programmes

What’s the consumer’s responsibility? *To inform regulators and industry of their concerns regarding food quality and safety *To follow relevant instructions and appropriate food hygiene measures How do they meet this responsibility? * Through constructive participation of consumer organizations in food standardisation work * Through consumer education

Import/Export Food Control WHY Detentions and Rejections

FAO Global Import Detention Study* n Few countries maintained records u Few countries made information available u Imported food requirements were not known u Import requirements were different u Inadequate communications amongst countries u Exporting Countries lack control measures u Confusing Certificates • Study conducted with the support of the Government of Finland






Import/Export Food Control n n Code of Ethics for International Trade in Food; and Principles for Food Import and Export Inspection and Certification

CODE OF ETHICS FOR INTERNATIONAL TRADE IN FOOD Principal objective: n n to stop exporting countries and exporters from dumping poor-quality or unsafe food on to international markets Currently being revised by the CCGP

Adopted Texts: Food Import and Export Inspection and Certification Systems n n n Guidelines for the Exchange of Information in Food Control Emergency Situations (CAC/GL 19 -1995) Principles for Food Import and Export Inspection and Certification (CAC/GL 20 -1995) Guidelines for the Exchange of Information Between Countries on Rejections of Imported Food (CAC/GL 25 -1997)

Adopted Texts: Food Import and Export Inspection and Certification Systems n n Guidelines for the Design, Operation, Assessment and Accreditation of Food Import and Export Inspection and Certification Systems (CAC/GL 26 -1997) Guidelines for the Development of Equivalence Agreements Regarding Food Import and Export Inspection and Certification Systems (CAC/GL 34 -1999)

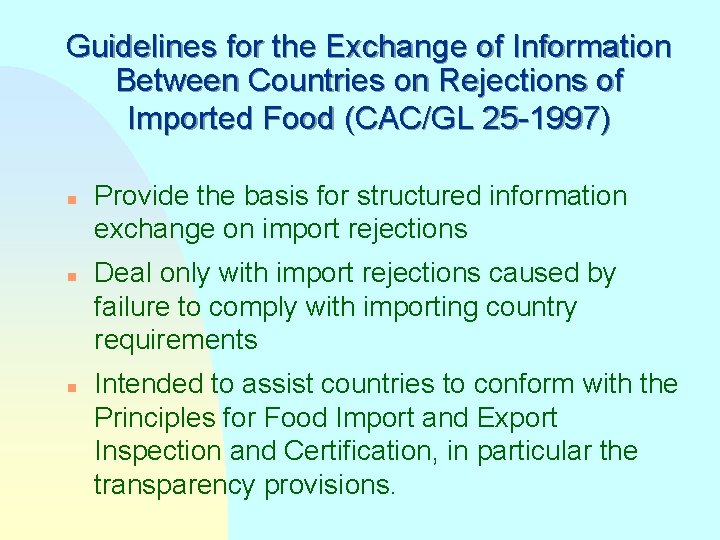
Guidelines for the Exchange of Information Between Countries on Rejections of Imported Food (CAC/GL 25 -1997) n n n Provide the basis for structured information exchange on import rejections Deal only with import rejections caused by failure to comply with importing country requirements Intended to assist countries to conform with the Principles for Food Import and Export Inspection and Certification, in particular the transparency provisions.

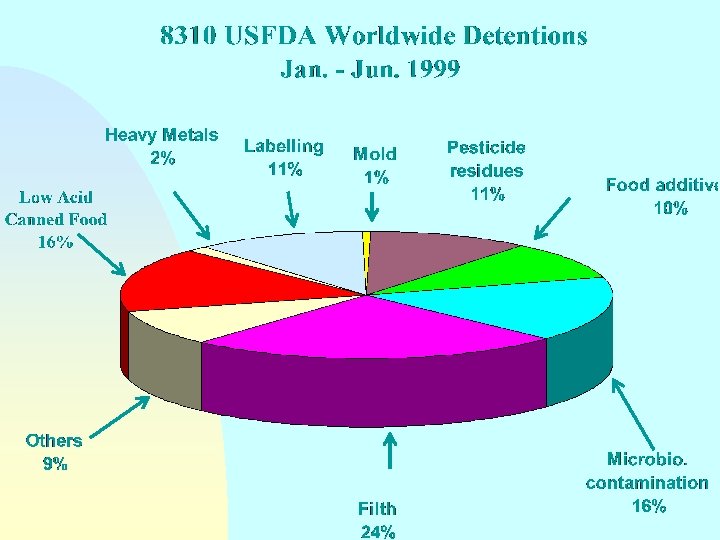
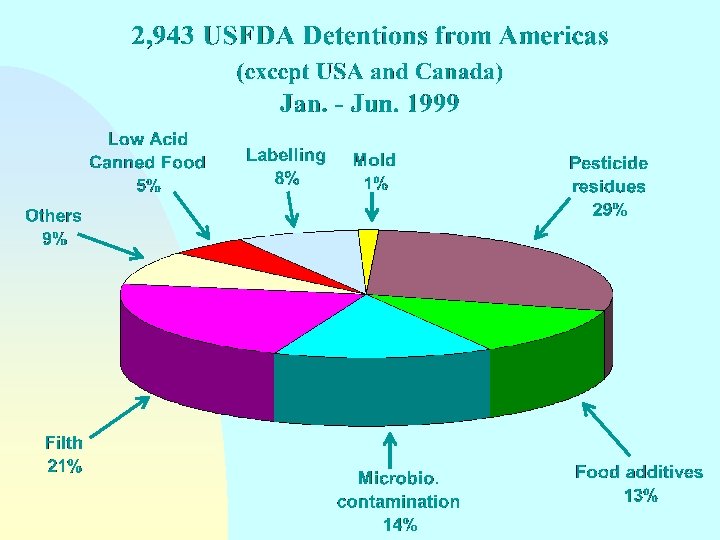
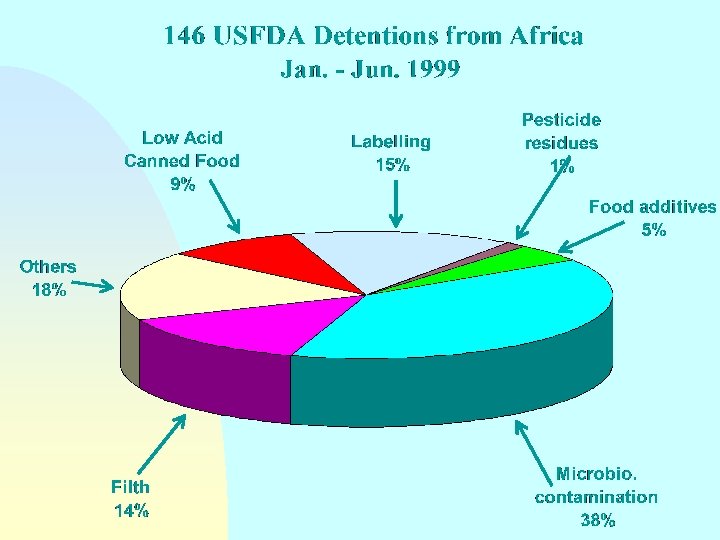
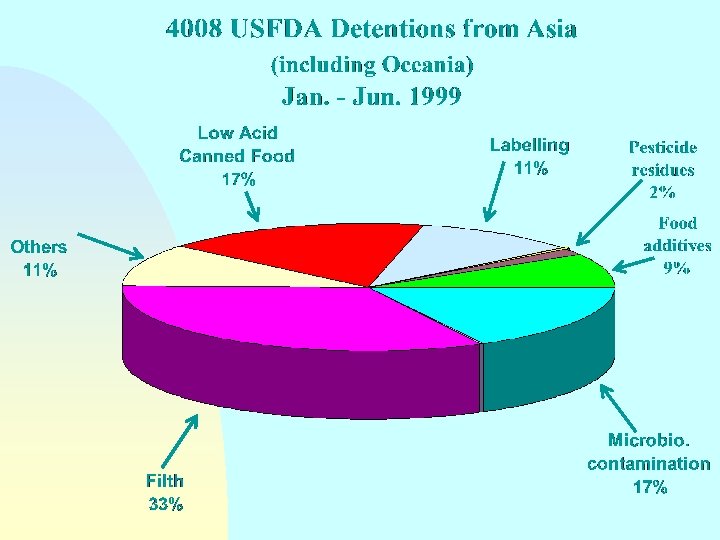
Guidelines for the Exchange of Information Between Countries on Rejections of Imported Food - Annex Reason(s) for rejection n Biological/microbiological contamination Chemical contamination (pesticide or veterinary drug residues, heavy metals, etc. ) Radionuclide contamination n Incorrect or misleading labelling n n Compositional defect Non-conformity with food additive requirements

Guidelines for the Exchange of Information Between Countries on Rejections of Imported Food - Annex Reason(s) for rejection n Organoleptic quality unacceptable n Technical or physical defects (e. g. packaging damage) n n n Incomplete or incorrect certification Does not come from an approved country, region or establishment Other reasons

Guidelines for the Exchange of Information Between Countries on Rejections of Imported Food - Annex Reason(s) for rejection NOTE: Where imported food has been rejected on the basis of sampling and/or analysis in the importing country, details should be made available on request as to sampling and analytical methods and test results and the identity of the testing laboratory.

Conference on International Food Trade Beyond 2000, 11 -15 October 1999, Melbourne General Recommendations of the Conference: n n called upon Member Governments to strengthen their contributions and participation in Codex work. called on countries to adhere to the FAO/WHO Code of Ethics for International Trade in Food in order to ensure that food products exported to developing countries meet national and international requirements.

Conference on International Food Trade Beyond 2000, 11 -15 October 1999, Melbourne General Recommendations of the Conference: n n governments should clearly acknowledge the role of consumers, producers and their representative bodies in the development of national and international food standards. Governments of member countries should take all necessary steps to apply Codex standards to all imported, exported and domestically produced and traded foods.

FUTURE WORK? n n n Establishing National Codex Committees Involvement in Codex work meetings, comments Harmonization of standards with Codex Cooperation between government, food industries and consumers Dedicated research in food

CONTACT ADDRESS: Joint FAO/WHO Food Standards Programme Food Quality and Standards Service FAO Viale delle Terme di Caracalla 00100 Rome, Italy Phone +39 (06) 57051 Fax +39 (06) 5705. 4593 Email: codex@fao. org http: //www. fao. org/es/esn/codex

Thank you