Famous scientists How many do you recognize Famous



- Slides: 12

Famous scientists: How many do you recognize?

Famous scientists: How many do you recognize?

Ernest Rutherford(30 August 1871 – 19 October 1937) World renowned physicist, the father of nuclear physics. Born in Nelson. The first person to discover the structure of the atom and the concept of half life.

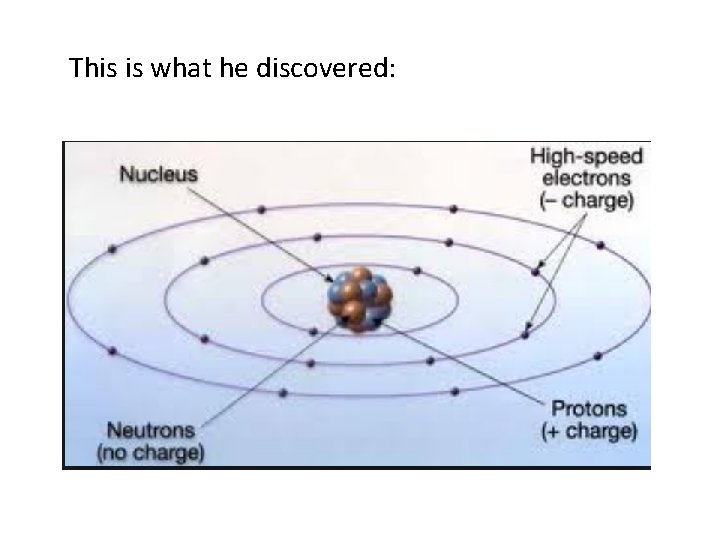
This is what he discovered:

Some facts: Everything around us is made of tiny wee things called atoms, they are so fine that we cannot see them. A tiny drop of water contain approximately 100000000000 atoms! Atoms come in different types and substances made up of only one type of atom is called elements. Scientists have discovered 118 different elements so far. Each element comes with a name and a symbol with numbers beside them, these elements are arranged in a table which is called the periodic table.

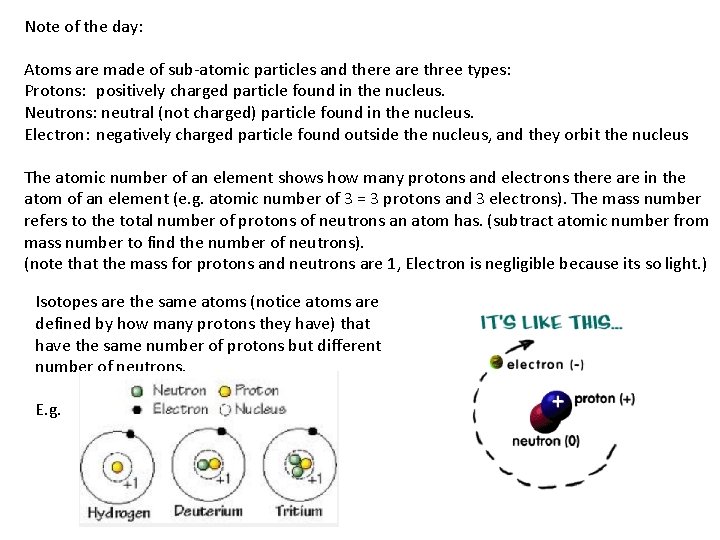
Note of the day: Atoms are made of sub-atomic particles and there are three types: Protons: positively charged particle found in the nucleus. Neutrons: neutral (not charged) particle found in the nucleus. Electron: negatively charged particle found outside the nucleus, and they orbit the nucleus The atomic number of an element shows how many protons and electrons there are in the atom of an element (e. g. atomic number of 3 = 3 protons and 3 electrons). The mass number refers to the total number of protons of neutrons an atom has. (subtract atomic number from mass number to find the number of neutrons). (note that the mass for protons and neutrons are 1, Electron is negligible because its so light. ) Isotopes are the same atoms (notice atoms are defined by how many protons they have) that have the same number of protons but different number of neutrons. E. g.

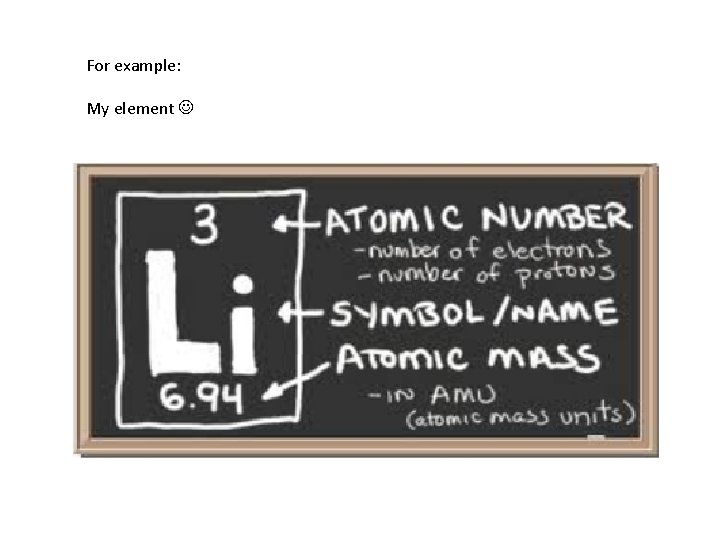
For example: My element

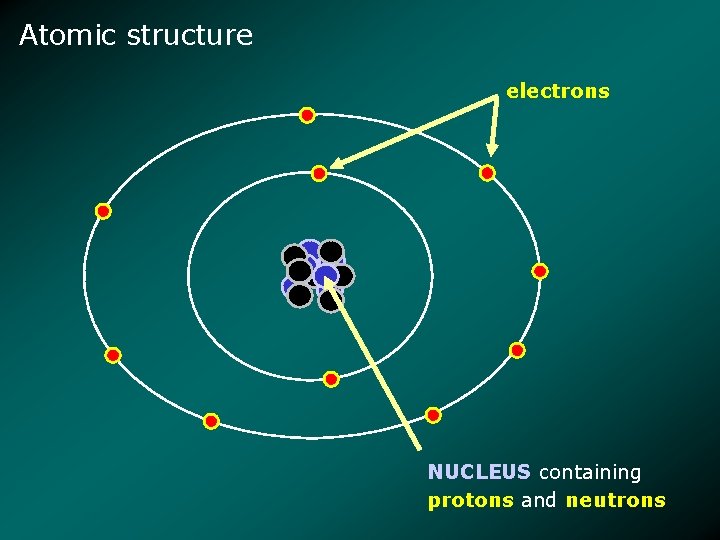
Atomic structure electrons NUCLEUS containing protons and neutrons

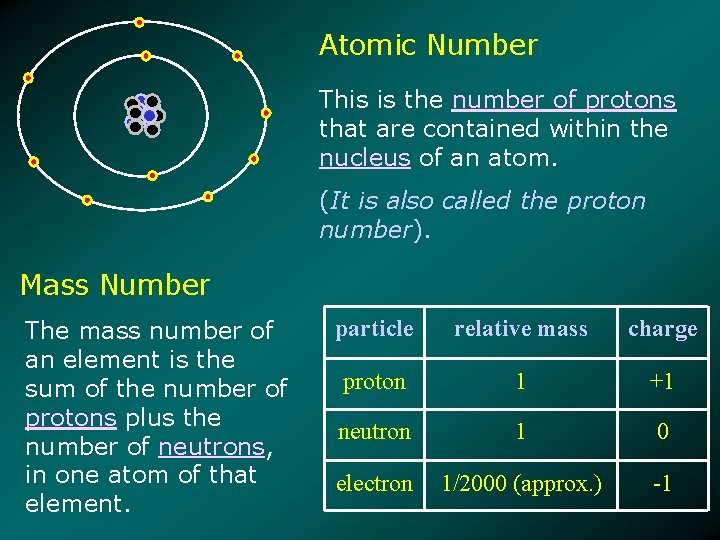
Atomic Number This is the number of protons that are contained within the nucleus of an atom. (It is also called the proton number). Mass Number The mass number of an element is the sum of the number of protons plus the number of neutrons, in one atom of that element. particle relative mass charge proton 1 +1 neutron 1 0 electron 1/2000 (approx. ) -1

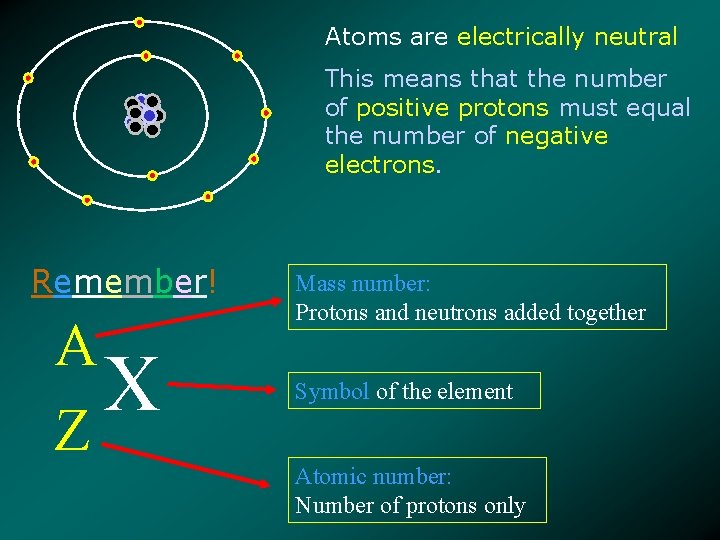
Atoms are electrically neutral This means that the number of positive protons must equal the number of negative electrons. Remember! A X Z Mass number: Protons and neutrons added together Symbol of the element Atomic number: Number of protons only

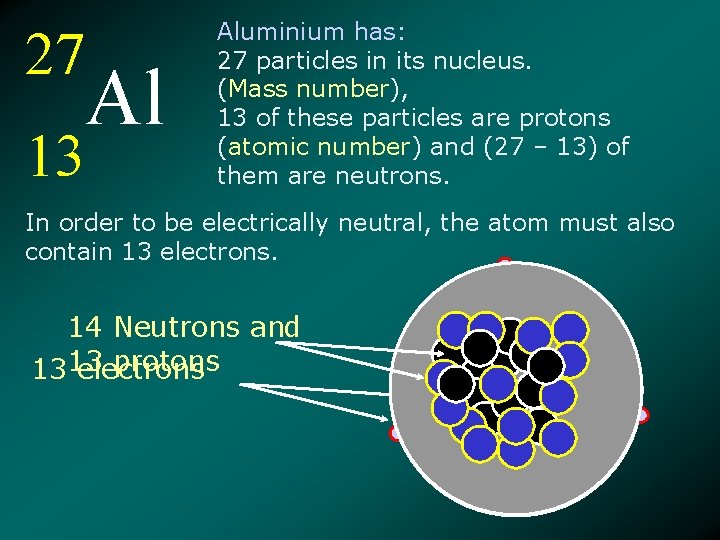
27 Al 13 Aluminium has: 27 particles in its nucleus. (Mass number), 13 of these particles are protons (atomic number) and (27 – 13) of them are neutrons. In order to be electrically neutral, the atom must also contain 13 electrons. 14 Neutrons and protons 1313 electrons

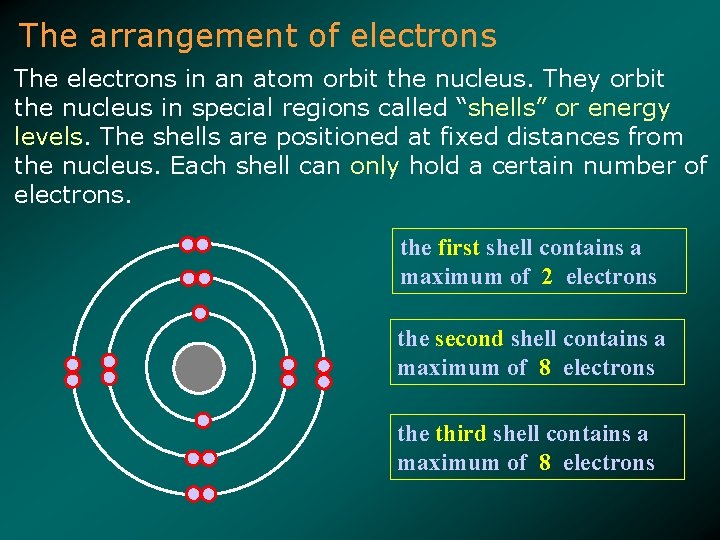
The arrangement of electrons The electrons in an atom orbit the nucleus. They orbit the nucleus in special regions called “shells” or energy levels. The shells are positioned at fixed distances from the nucleus. Each shell can only hold a certain number of electrons. the first shell contains a maximum of 2 electrons the second shell contains a maximum of 8 electrons the third shell contains a maximum of 8 electrons