Fallout Spark 0 1 e Strontium 90 spontaneously









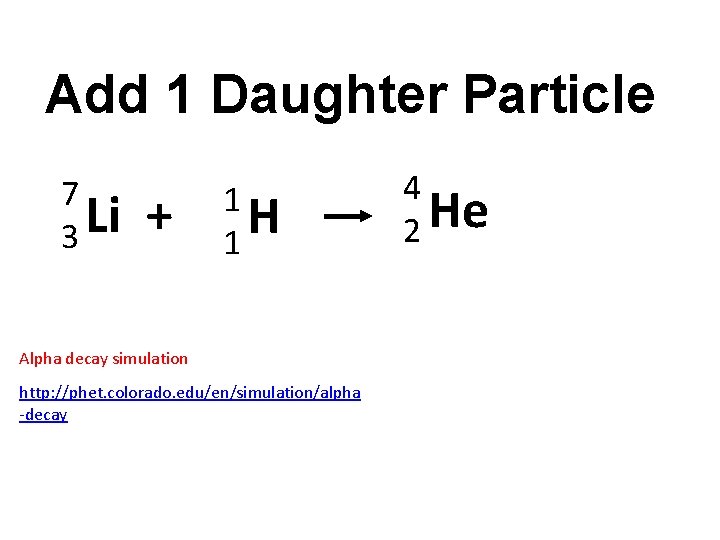
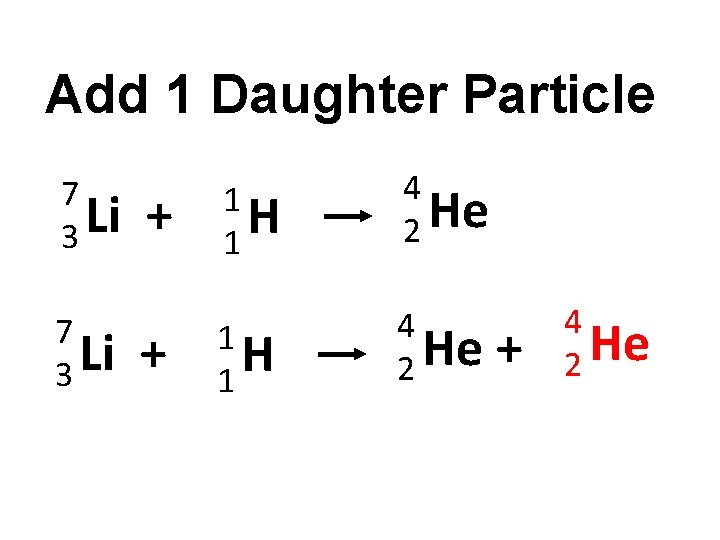
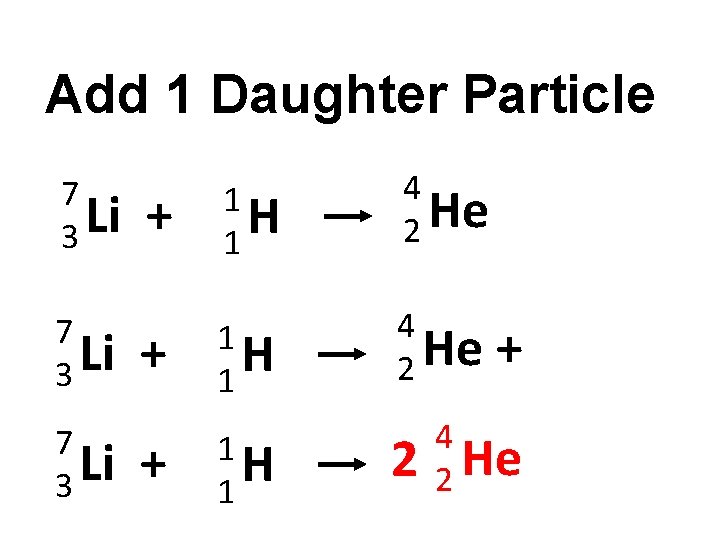
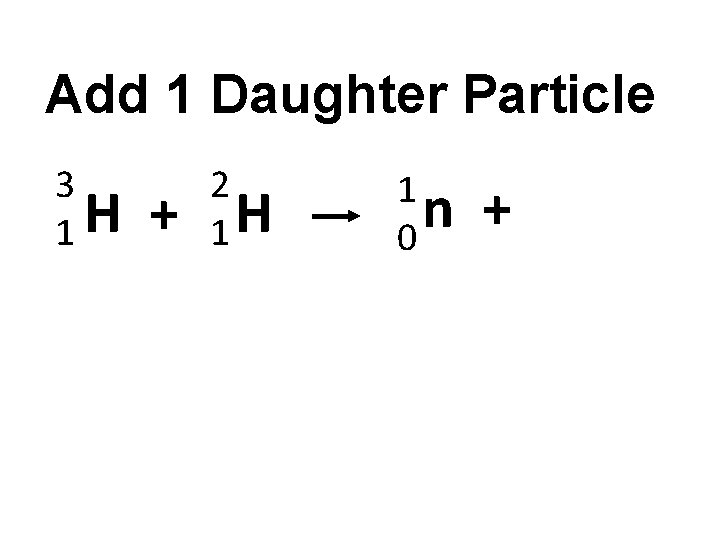

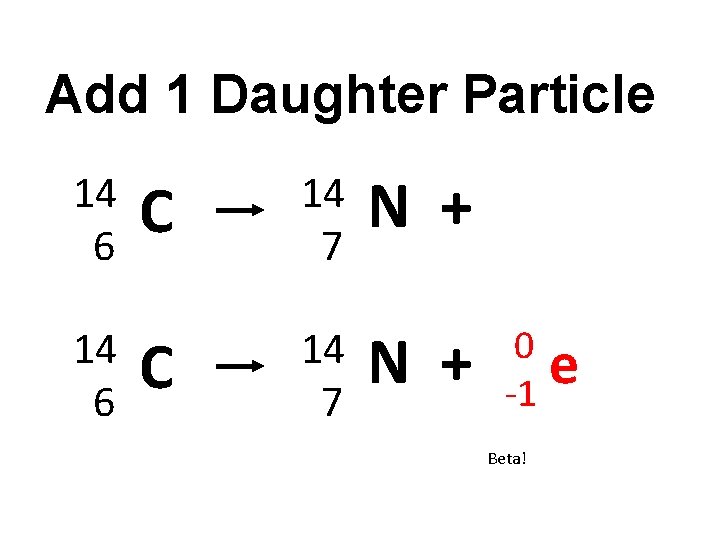
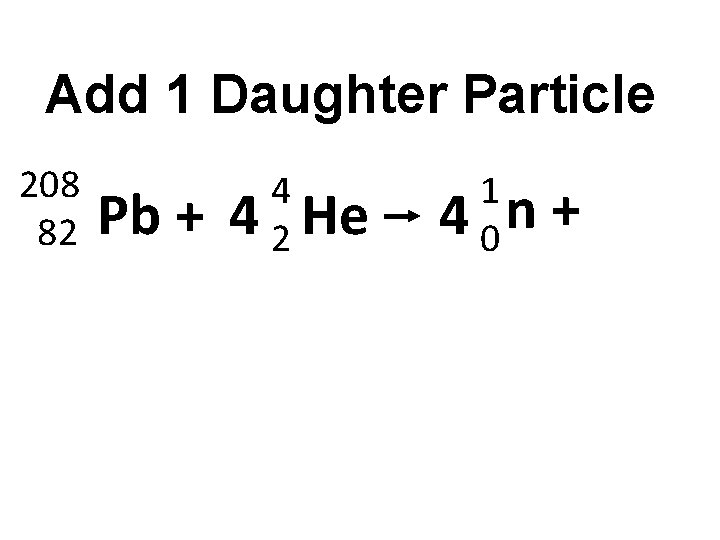
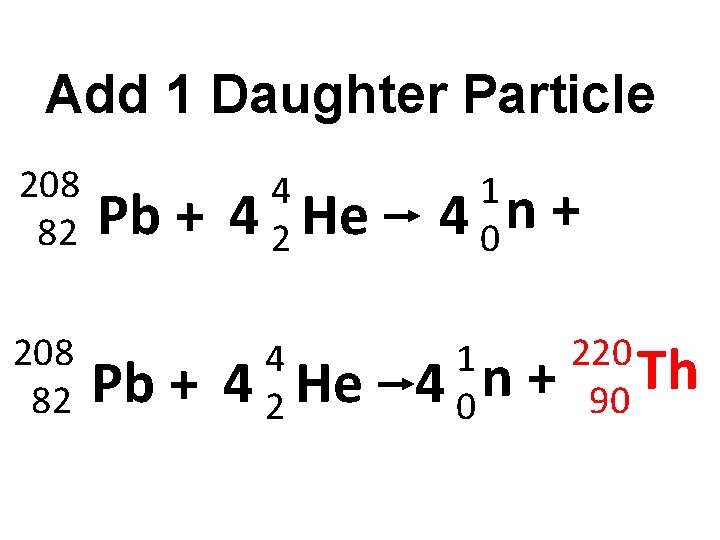


- Slides: 48

Fallout Spark 0 -1 e Strontium – 90 spontaneously decays and releases a beta particle. What is the identity of the new isotope? (It is bad news because Sr is in the same chemical group as Ca) Write the balanced nuclear equation. It has a half-life of 28. 8 years meaning that half of an initial amount of Sr-90 will remain after 28 years. How much of an original amount of 35 grams will be left after 3 half-lives. How much is left over? How much time passed?
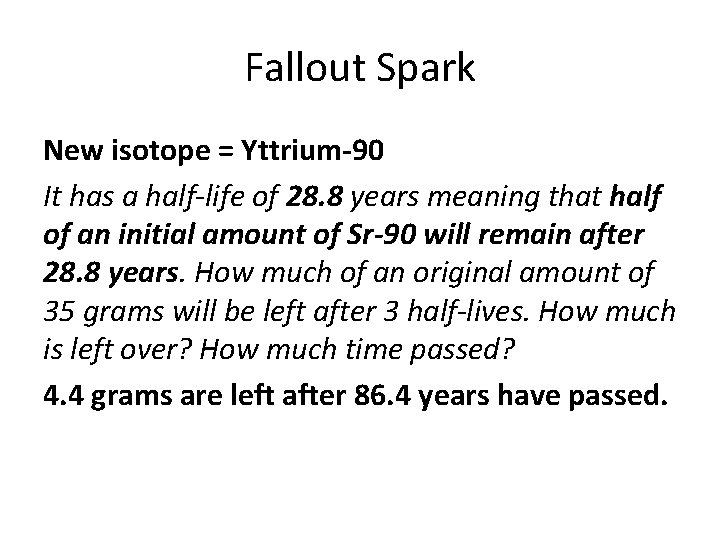
Fallout Spark New isotope = Yttrium-90 It has a half-life of 28. 8 years meaning that half of an initial amount of Sr-90 will remain after 28. 8 years. How much of an original amount of 35 grams will be left after 3 half-lives. How much is left over? How much time passed? 4. 4 grams are left after 86. 4 years have passed.

Speaking of MASS DEFECT… Frank’s sleeves were missing… They were found at the gun show!

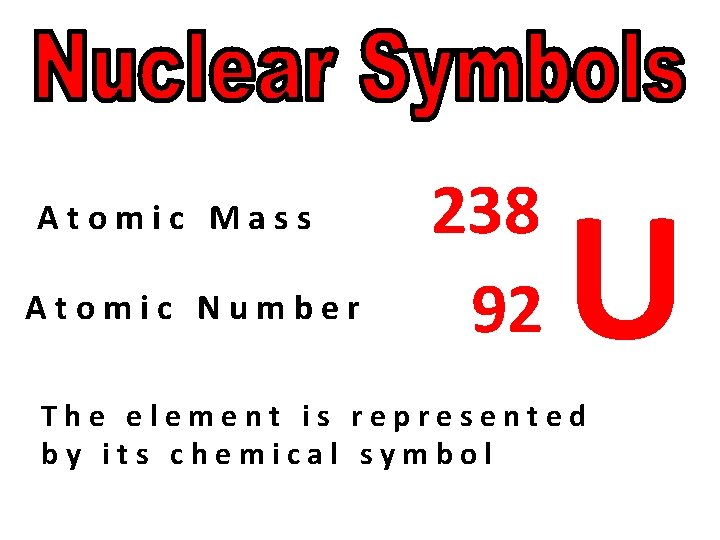
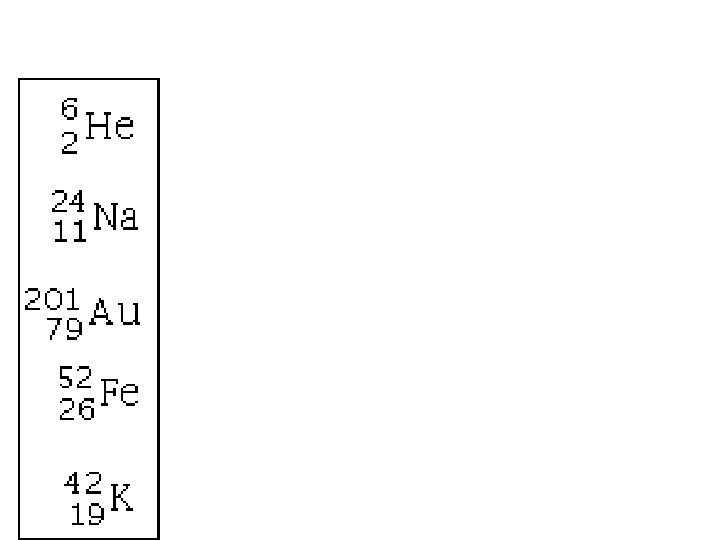
Atomic Mass Atomic Number 238 92 U The element is represented by its chemical symbol

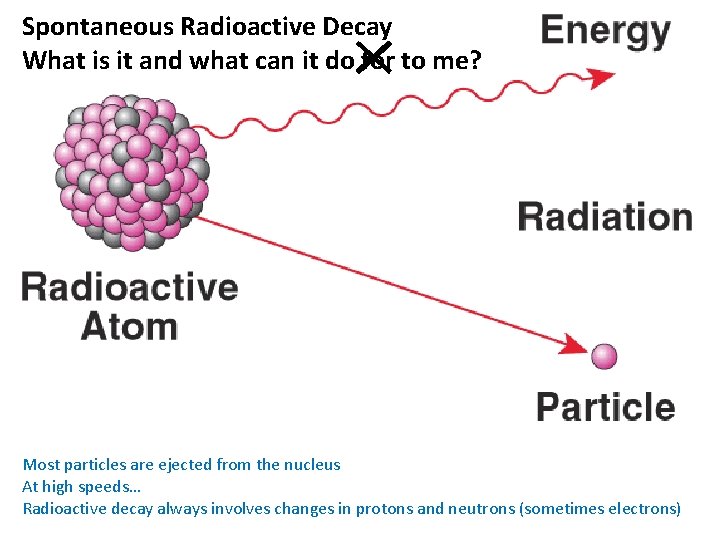
Spontaneous Radioactive Decay What is it and what can it do for to me? Most particles are ejected from the nucleus At high speeds… Radioactive decay always involves changes in protons and neutrons (sometimes electrons)

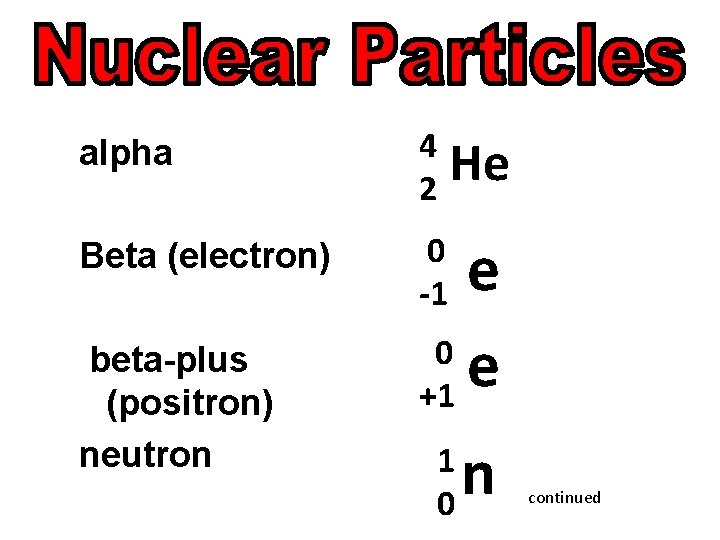
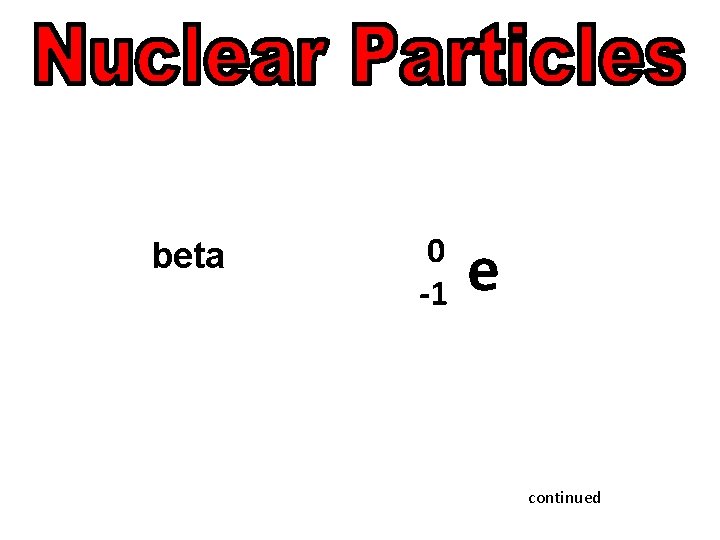
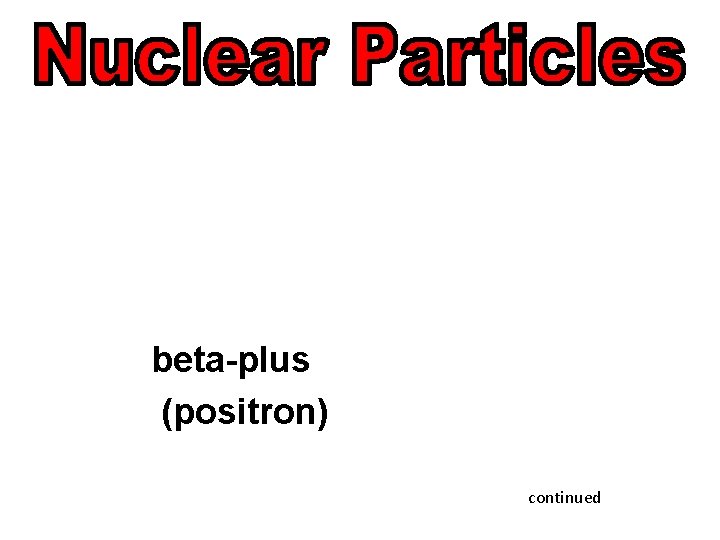
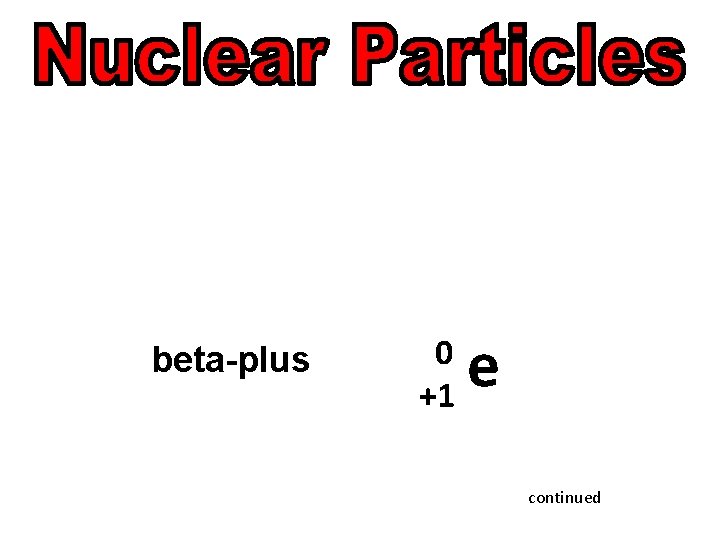
alpha 4 2 Beta (electron) 0 -1 beta-plus (positron) neutron He e 0 e +1 1 0 n continued

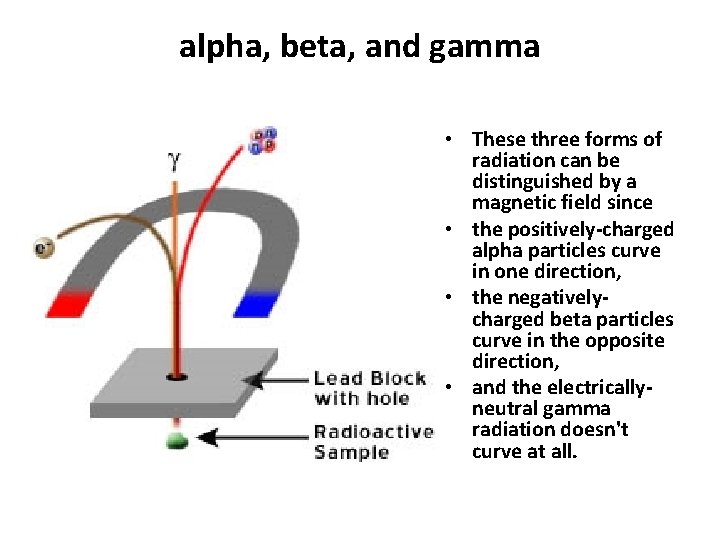
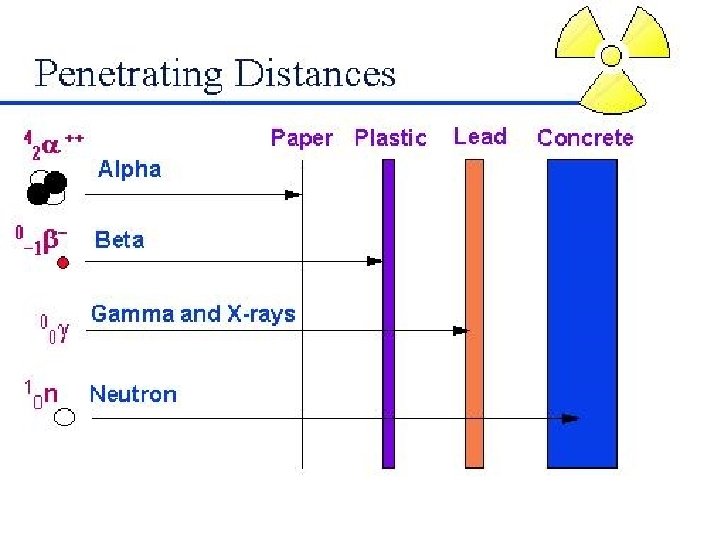
alpha, beta, and gamma • These three forms of radiation can be distinguished by a magnetic field since • the positively-charged alpha particles curve in one direction, • the negativelycharged beta particles curve in the opposite direction, • and the electricallyneutral gamma radiation doesn't curve at all.

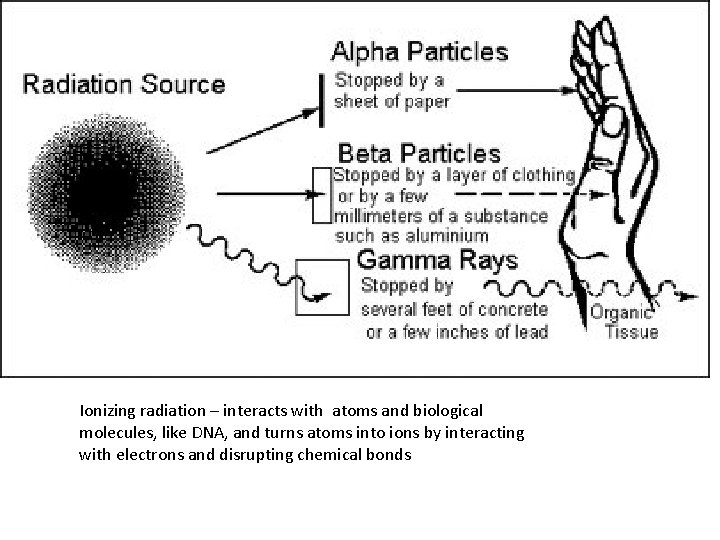
Ionizing radiation – interacts with atoms and biological molecules, like DNA, and turns atoms into ions by interacting with electrons and disrupting chemical bonds


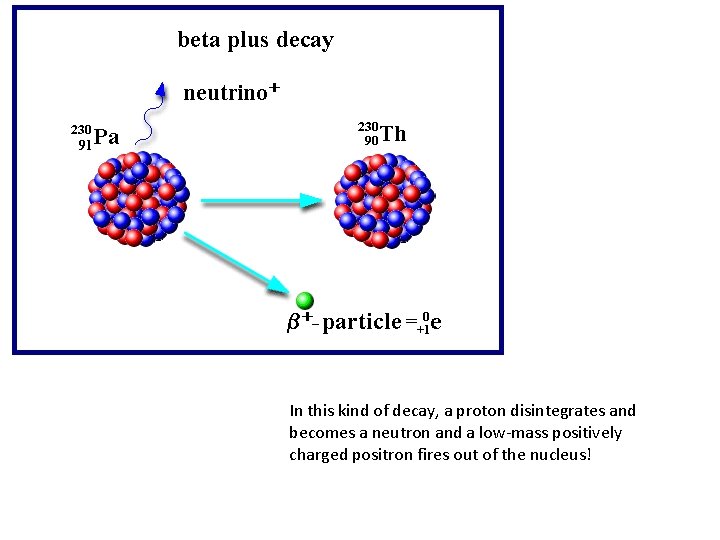
In this kind of decay, a proton disintegrates and becomes a neutron and a low-mass positively charged positron fires out of the nucleus!

Nuclear equations are balanced much like chemical equations.

To balance nuclear equations, follow these two rules: - mass number is conserved - electric charge is conserved

Rule One Mass number is conserved: The sum of the mass numbers before the change must equal the sum of the mass numbers after the change.

Rule Two Charge is conserved: The total electric charge on subatomic particles and nuclei before and after the change must be equal.

alpha continued

alpha 4 2 He continued

beta continued

beta 0 -1 e continued

beta-plus (positron) continued

beta-plus 0 +1 e continued

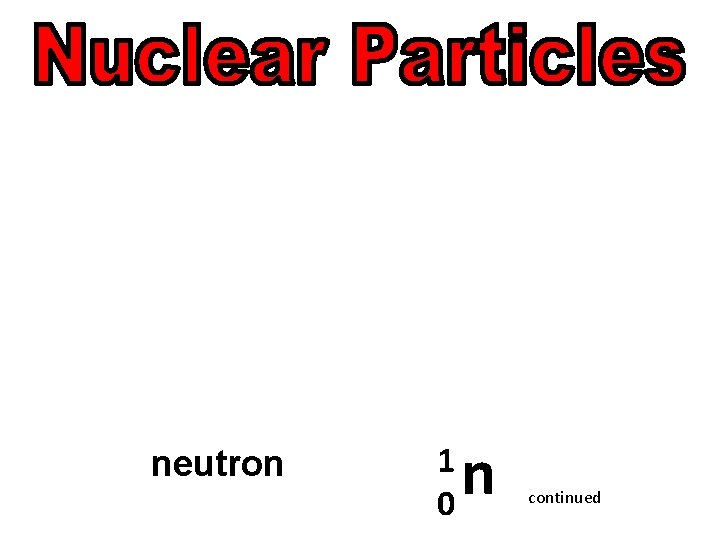
neutron continued

neutron 1 0 n continued

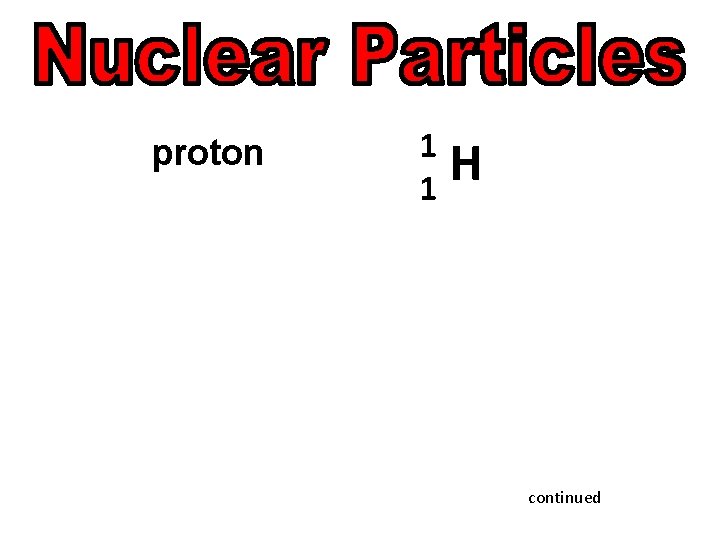
proton continued

proton 1 1 H continued
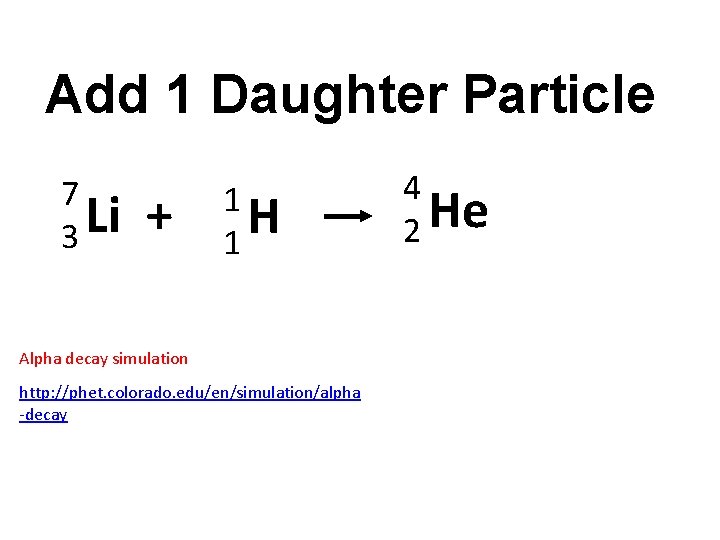
Add 1 Daughter Particle 7 Li 3 + 1 H 1 Alpha decay simulation http: //phet. colorado. edu/en/simulation/alpha -decay 4 He 2
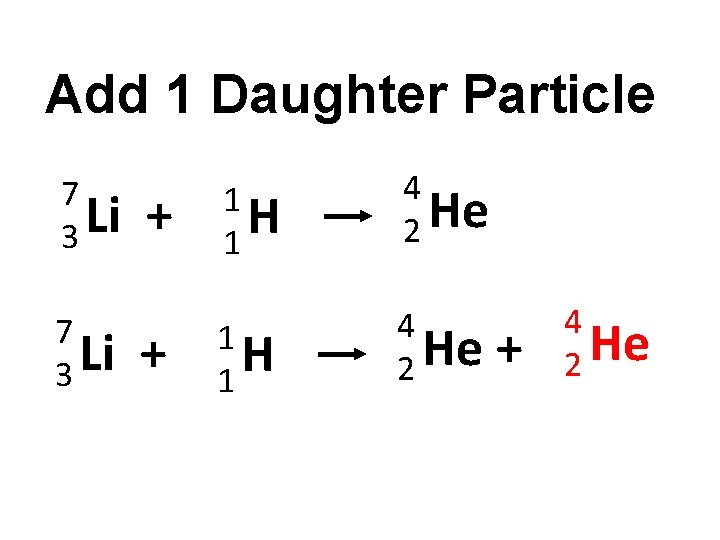
Add 1 Daughter Particle 7 Li 3 + + 1 H 1 4 He 2 + 4 2 He
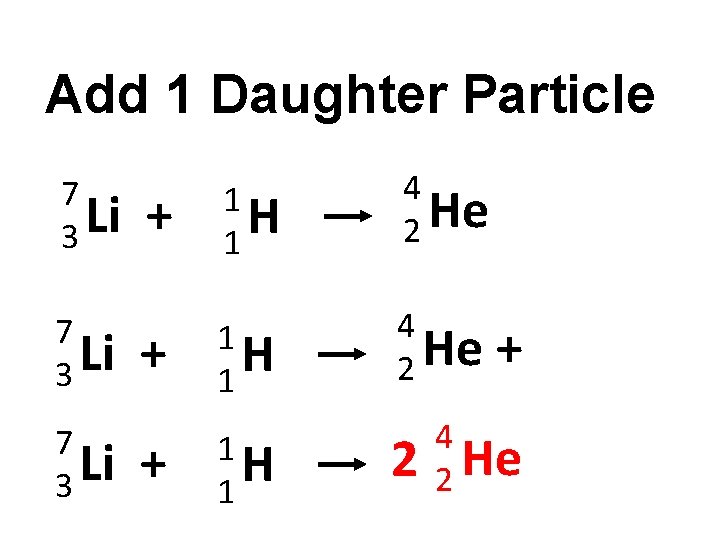
Add 1 Daughter Particle 7 Li 3 1 H 1 4 He 2 + 1 H 1 + 2 + 4 He 2 4 2 He
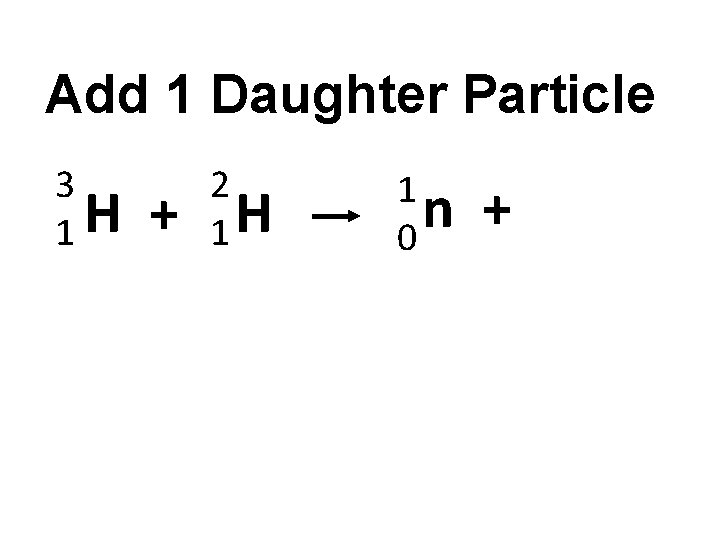
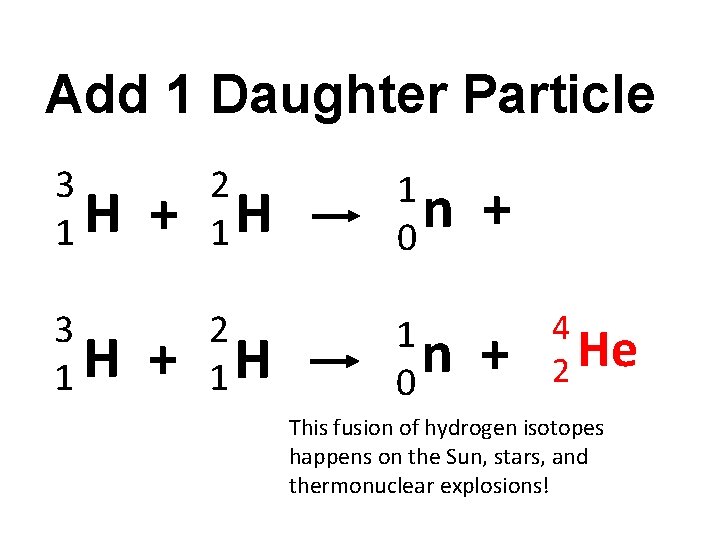
Add 1 Daughter Particle 3 H 1 + 2 1 H 1 n 0 +

Add 1 Daughter Particle 3 H 1 3 1 H + 2 1 H 1 n 0 + + 4 He 2 This fusion of hydrogen isotopes happens on the Sun, stars, and thermonuclear explosions!

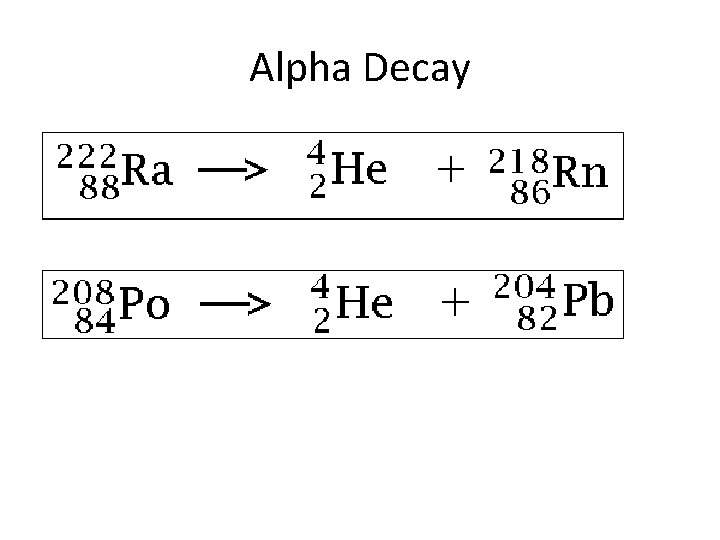
Alpha Decay


What particle is ejected from the unstable nucleus of carbon-14? 14 6 C 14 7 N + Describe what happens to the nucleus… http: //phet. colorado. edu/en/simulation/betadecay
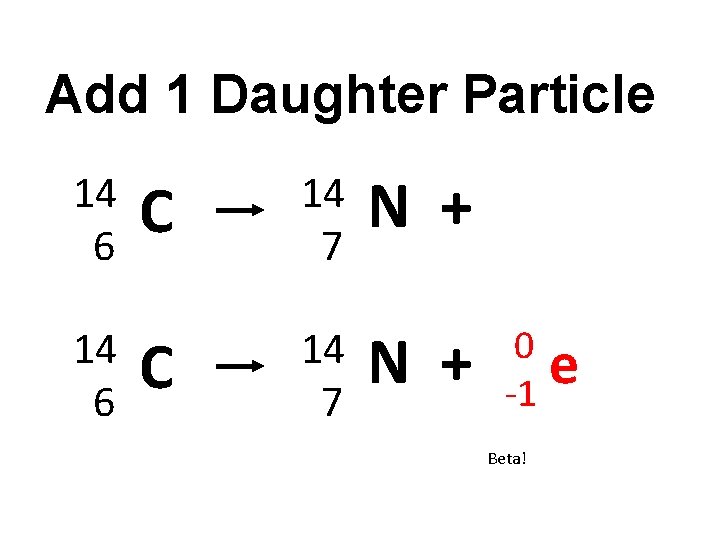
Add 1 Daughter Particle 14 6 C 14 7 N + 0 e -1 Beta!

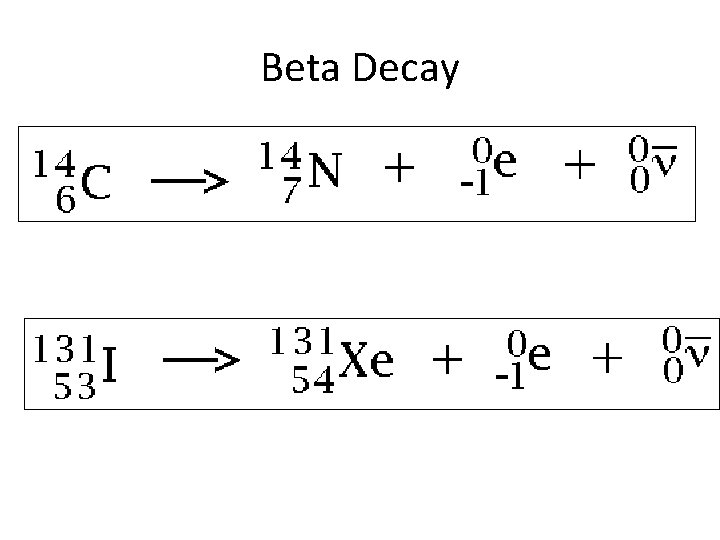
Beta Decay


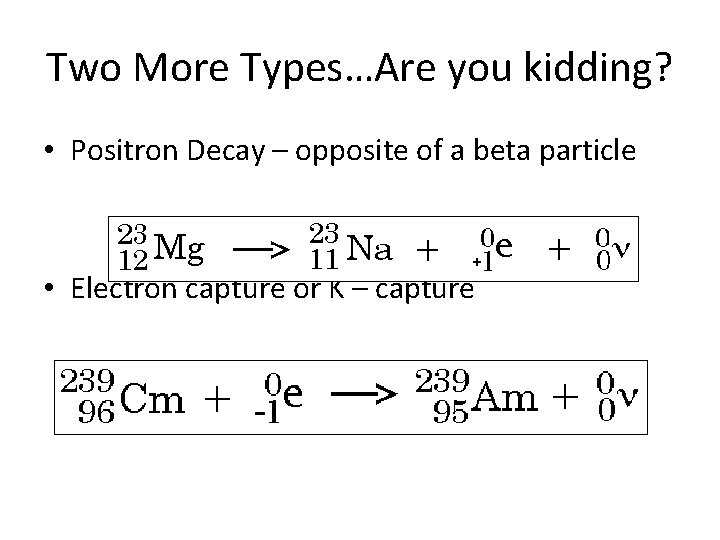
Two More Types…Are you kidding? • Positron Decay – opposite of a beta particle • Electron capture or K – capture
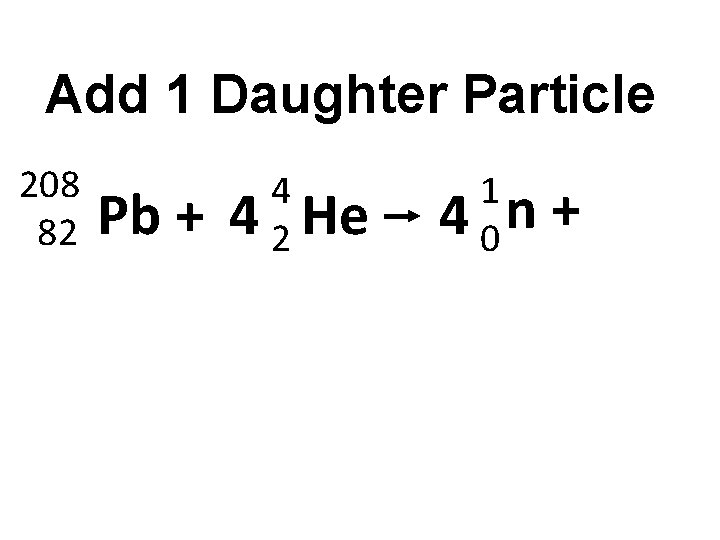
Add 1 Daughter Particle 208 82 Pb + 4 4 2 He 1 4 0 n +
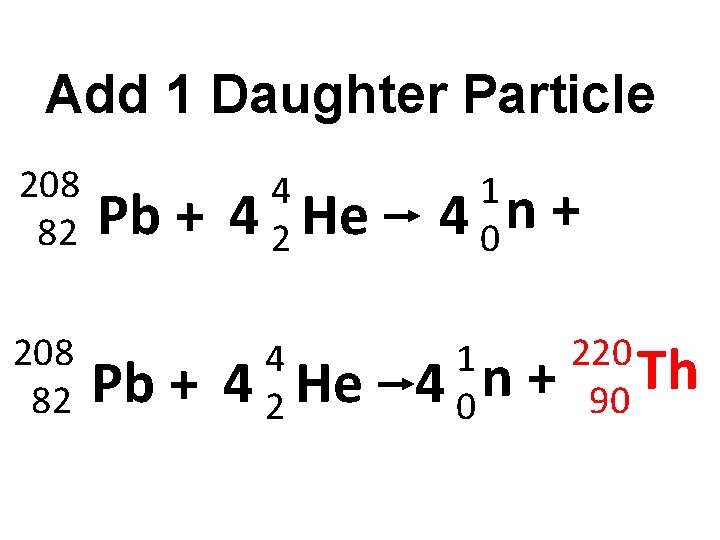
Add 1 Daughter Particle 208 82 Pb + 4 4 2 He 1 4 0 n + + 220 Th 90

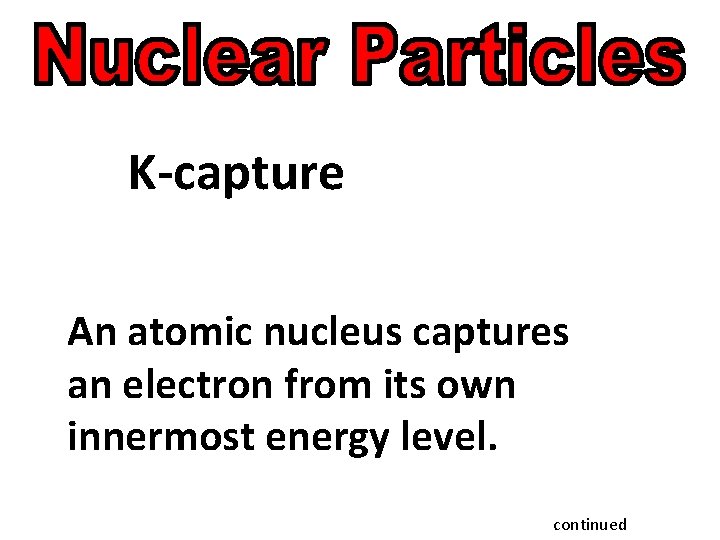
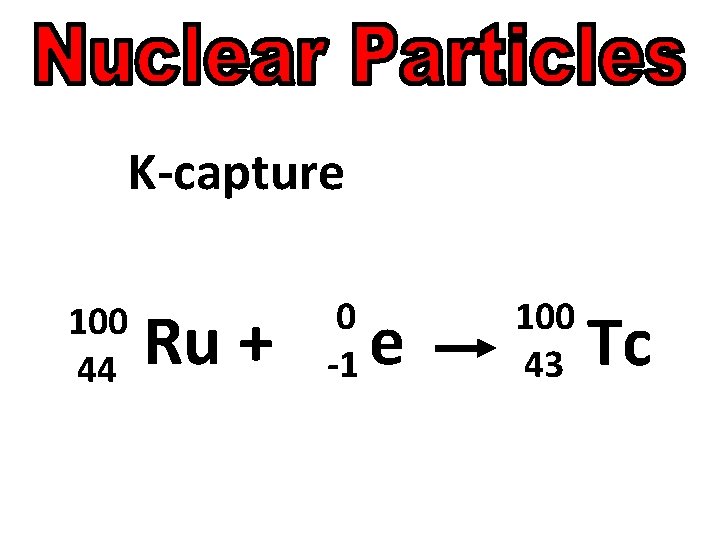
K-capture An atomic nucleus captures an electron from its own innermost energy level. continued

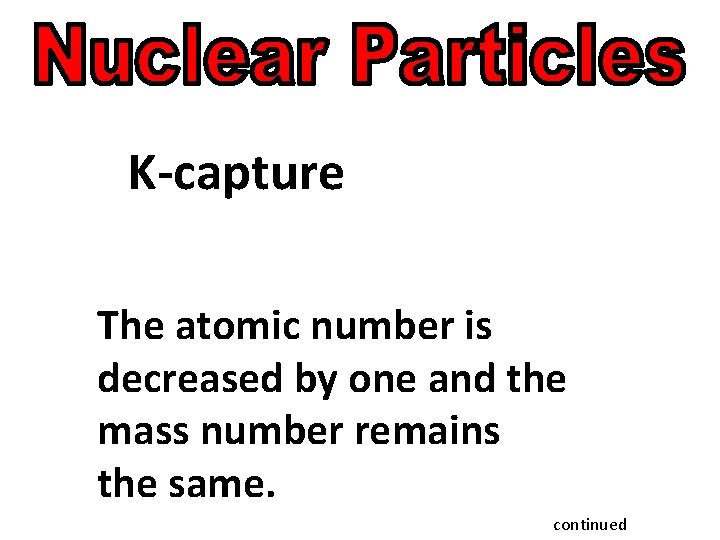
K-capture The atomic number is decreased by one and the mass number remains the same. continued

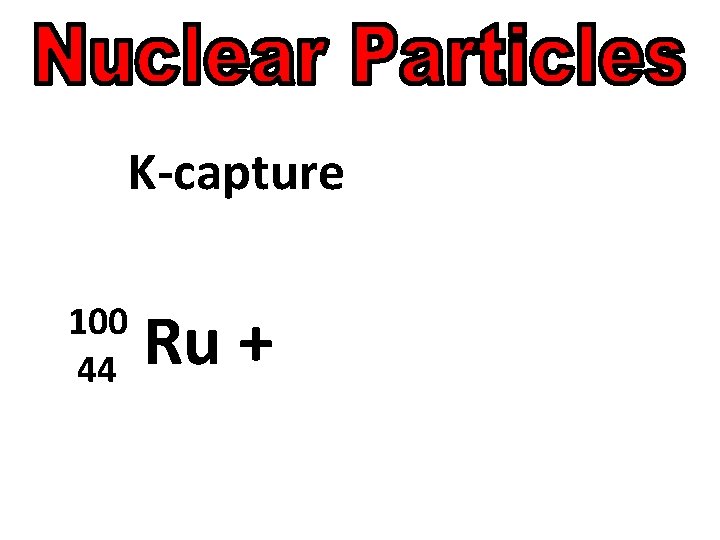
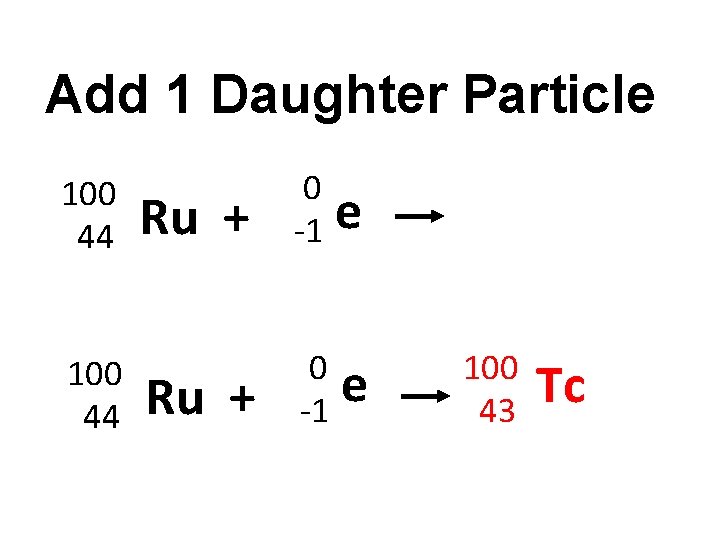
K-capture 100 44 Ru +

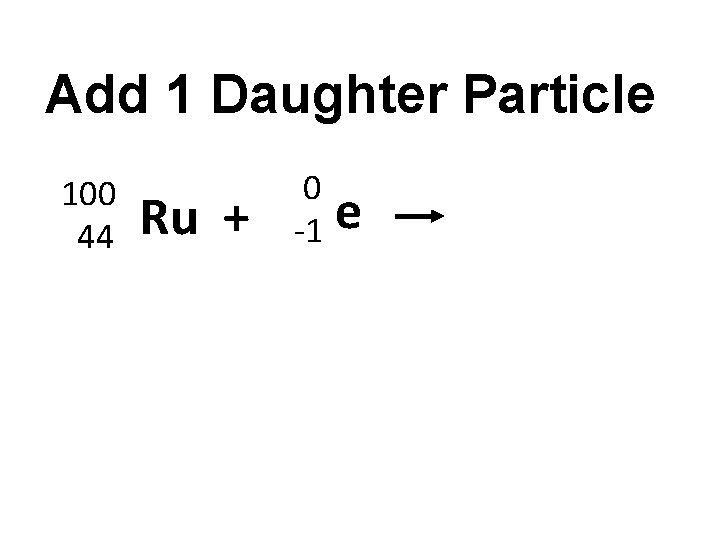
Add 1 Daughter Particle 100 44 Ru + 0 -1 e

Add 1 Daughter Particle 100 44 Ru + 0 -1 e e 100 43 Tc

K-capture 100 44 Ru + 0 -1 e 100 43 Tc

You Try! • Complete the worksheet on balancing nuclear equations. • Can you describe what is happening to the nuclear particles with each type of nuclear change? You should.

Strontium-90 Question • Strontium-90 is a radioactive isotope that decays via a beta particle. It has a half-life of 28. 8 years. Chemically it behaves like calcium and will be absorbed into bones and can potentially lead to bone cancer or leukemia. Recall that Sr-90 becomes Y-90 via beta decay. • Use your notes packet and find the half-life equation for the following problem: • 500 years from now, how many grams of a 0. 0256 kg sample of strontium – 90 will remain? How much Y 90 will be created over the same time span?

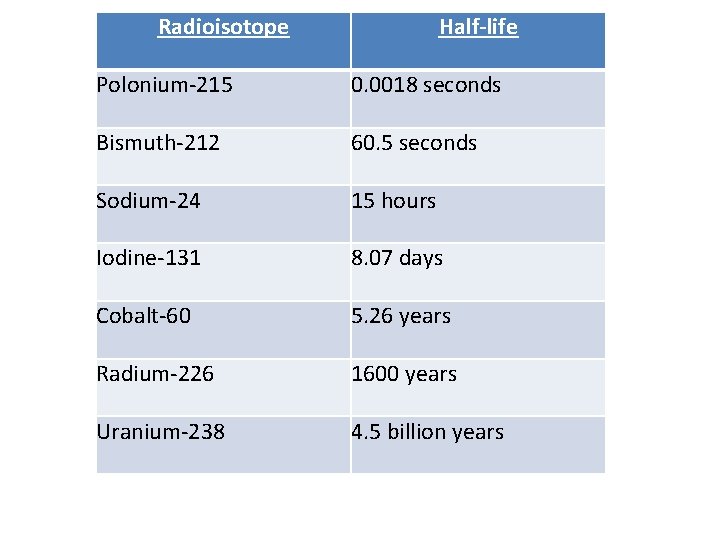
Radioisotope Half-life Polonium-215 0. 0018 seconds Bismuth-212 60. 5 seconds Sodium-24 15 hours Iodine-131 8. 07 days Cobalt-60 5. 26 years Radium-226 1600 years Uranium-238 4. 5 billion years

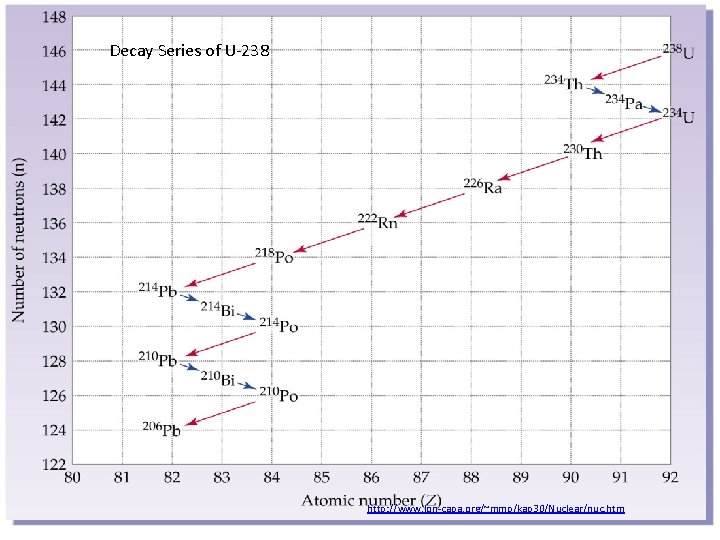
Decay Series of U-238 http: //www. lon-capa. org/~mmp/kap 30/Nuclear/nuc. htm