Facts for Ionic Formulas Simple Ionic Compounds Concept





- Slides: 12

Facts for Ionic Formulas

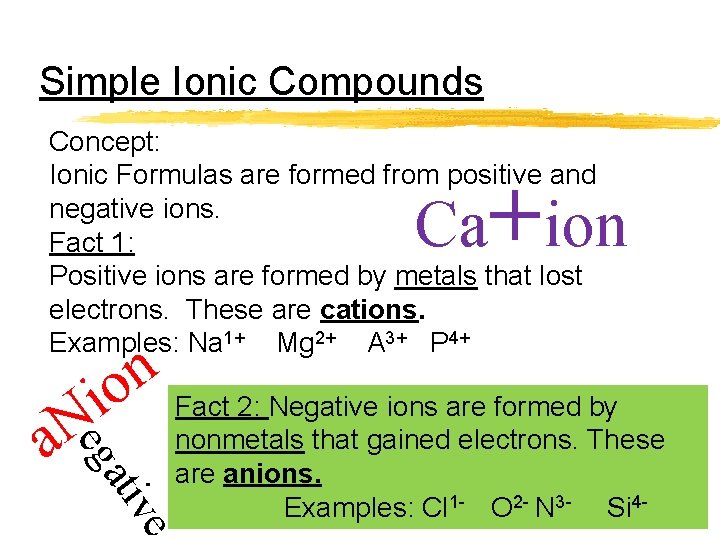
Simple Ionic Compounds Concept: Ionic Formulas are formed from positive and negative ions. Fact 1: Positive ions are formed by metals that lost electrons. These are cations. Examples: Na 1+ Mg 2+ A 3+ P 4+ Ca+ion n io Fact 2: Negative ions are formed by eg N a ati ve nonmetals that gained electrons. These are anions. Examples: Cl 1 - O 2 - N 3 - Si 4 -

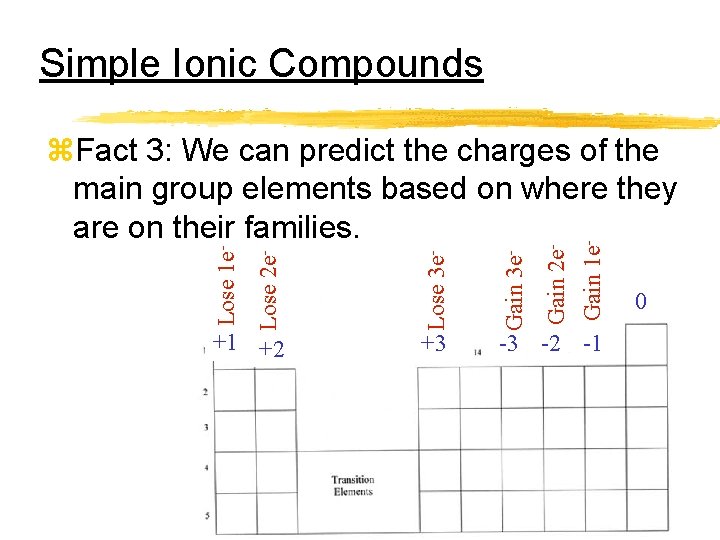
Simple Ionic Compounds +3 Gain 2 e. Gain 1 e- Gain 3 e- +1 +2 Lose 3 e- Lose 2 e- Lose 1 e- z. Fact 3: We can predict the charges of the main group elements based on where they are on their families. -3 -2 -1 0

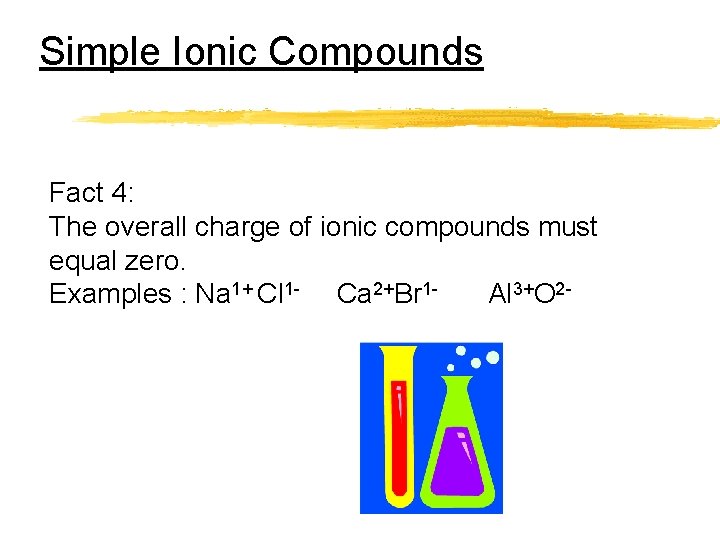
Simple Ionic Compounds Fact 4: The overall charge of ionic compounds must equal zero. Examples : Na 1+ Cl 1 - Ca 2+Br 1 Al 3+O 2 -

Lesson Two--Polyatomic Ion Formulas Concept: Polyatomic ions are groups of atoms that behave as one unit. Fact 5: Some ions have more that one atom. You will need to memorize most of these but some you can figure out from their atoms. Examples: (SO 4) = S +6 and O -8 = -2 (NO 3)= N +5 and O -6 = -1 (NH 4) = N -3 and H +4 = +1

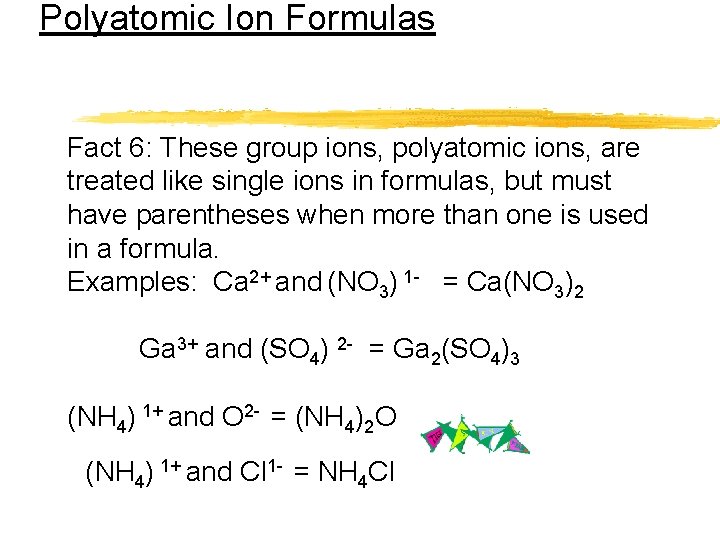
Polyatomic Ion Formulas Fact 6: These group ions, polyatomic ions, are treated like single ions in formulas, but must have parentheses when more than one is used in a formula. Examples: Ca 2+ and (NO 3) 1 - = Ca(NO 3)2 Ga 3+ and (SO 4) 2 - = Ga 2(SO 4)3 (NH 4) 1+ and O 2 - = (NH 4)2 O (NH 4) 1+ and Cl 1 - = NH 4 Cl

Polyatomic Ion Formulas Fact 7: The polyatomic ions are named based on the atoms that they contain. Those with oxygen and another nonmetal are often name "____ate" with the root of the other nonmetal in the blank. Examples: (NO 3)1 - is nitrate (SO 4)2 - is sulfate (Cl. O 3) 1 - is chlorate

Polyatomic Ion Formulas Fact 8: Those polyatomic ions with one oxygen less than the "ate" ions are named "----ite" ions. Examples: (NO 2)1 - is nitrite (SO 3)2 - is sulfite (Cl. O 2)1 - is chlorite

Lesson Three--Transition Metal Compounds Concept: Transition metals have electrons in d orbitals and can donate different numbers of electrons, thus giving them several different positive charges. Fact 9: These can be determined from the Roman numeral which is written next to the metal's name. Example: Cu 1+is Copper I Pb 2+is Lead II Fe 3+is Iron III Sn 4+s Tin IV

Transition Metal Compounds Fact 10: These transition metals are used in formulas just like other metals, once the charge is determined from the Roman numeral in the name. Example: Cu 1+ Cl 1 - = Cu. Cl Pb 2+O 2 - = Pb. O Fe 3+Br 1 - = Fe. Br 3 Sn 4+O 2 - = Sn. O 2

Transition Metal Compounds Fact 11: A few transition metal ions only have one charge and never change so they can be written without a Roman numeral in their formula name. Example: Ag 1+ Zn 2+ Cd 2+

Lesson Four--Using Formulas in Problem Solving Concept: Correctly written chemical formulas hold a large amount of information for the prepared student to find. Fact 12: The subscripts tell us the number of atoms of each kind that is present in the compound. Example: Na. Cl has one atom of sodium and one atom of chlorine. H 2 SO 4 has two atoms of hydrogen, one atom of sulfur and four atoms of oxygen