Factors Affecting Solubility The Polarity of the Solute

Factors Affecting Solubility: The Polarity of the Solute and Solvent Learning Goal: I will be able to describe the properties of water that make it a good solvent for polar compounds I will explain the process of how soap works by using the terms hydrophobic and hydrophilic

General Rule • The general rule is "like dissolves like" • Polar substances dissolve polar substances • Non-polar substances dissolve nonpolar substances.

Importance of Water • More than 70% of our total body weight is water • Necessary for photosynthesis – H becomes incorporated into organic compounds – Oxygen released for us to breathe • Solvent for most biochemical reactions

Water is a polar molecule • Due to differences in electronegativities, water is slightly charged at its poles – Oxygen takes on a slight – charge – Hydrogens take on a slight + charge • http: //programs. northlandcollege. edu/biology/Bi ology 1111/animations/hydrogenbonds. html
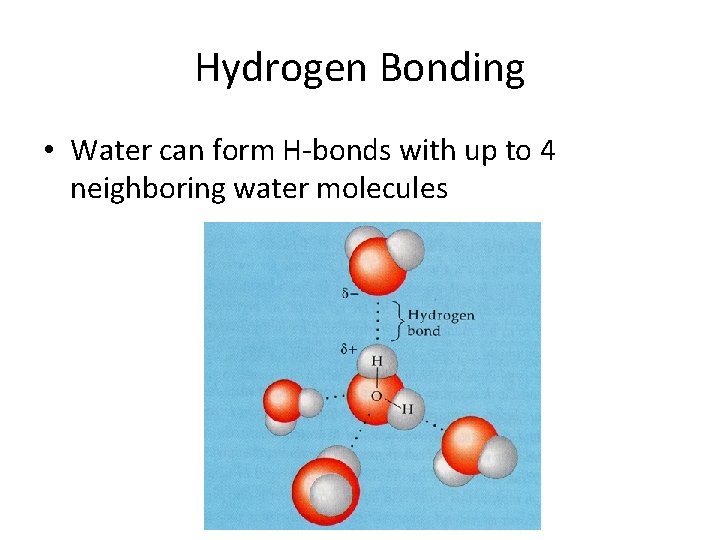
Hydrogen Bonding • Water can form H-bonds with up to 4 neighboring water molecules
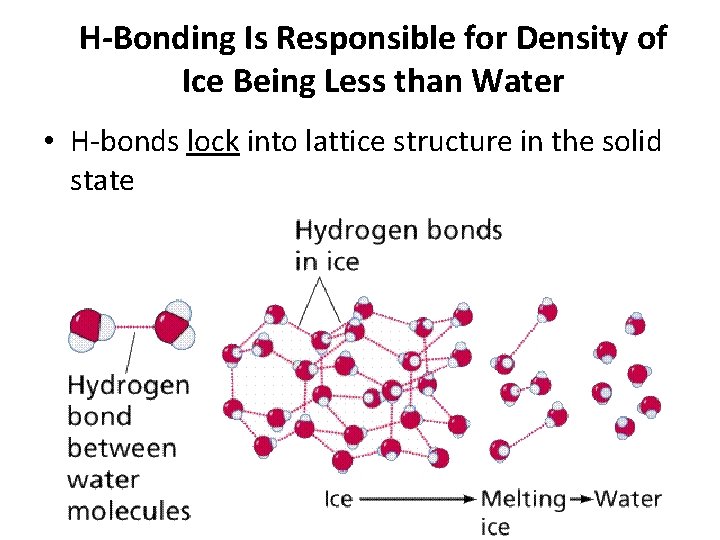
H-Bonding Is Responsible for Density of Ice Being Less than Water • H-bonds lock into lattice structure in the solid state

Ice • Solid water is less dense than liquid water, allowing it to float

Water is the universal solvent • Water can dissolve many hydrophilic (water loving) substances – Ionic compounds – Other polar compounds • Hydration is the process of water molecules surrounding ions to make “spheres of hydration” • http: //www. mhhe. com/physsci/chemistry/essen tialchemistry/flash/molvie 1. swf
Think/Pair/Share: • Can water dissolve non-polar oil? Why or why not?

• Some substances do not dissolve readily in water because they are non-polar solutes • Oil (fats or lipids) is an example of a hydrophobic – “water-fearing” - substance • Application: How does soap work to clean grease/oil from our dishes?

How soap works: micelles Soap can suspend oil/dirt in such a way that it can be removed
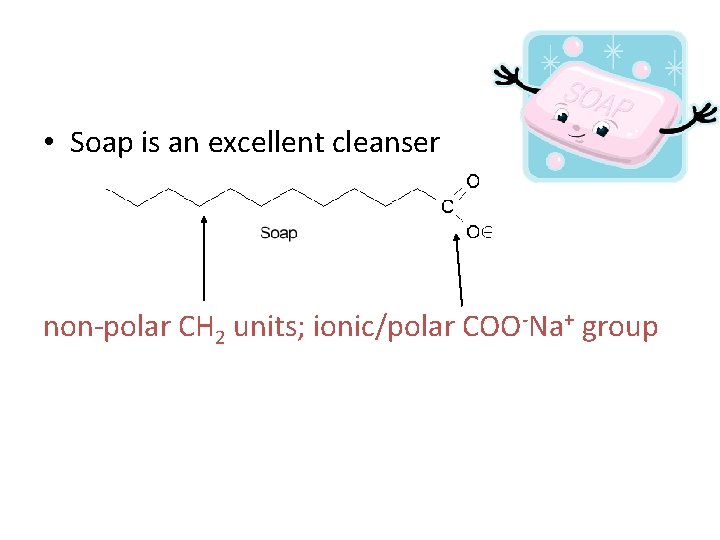
• Soap is an excellent cleanser non-polar CH 2 units; ionic/polar COO-Na+ group

• Grease and oil are non-polar and insoluble in HOH. • When soap added to oil-containing solution, the non-polar hydrocarbon portion of the soap attaches to the non-polar oil molecules.

• A micelle then forms—with nonpolar solutes (grease/oil) in the centre. • The outside of the micelle is polar (water soluble) and now the grease and oil can be washed away.
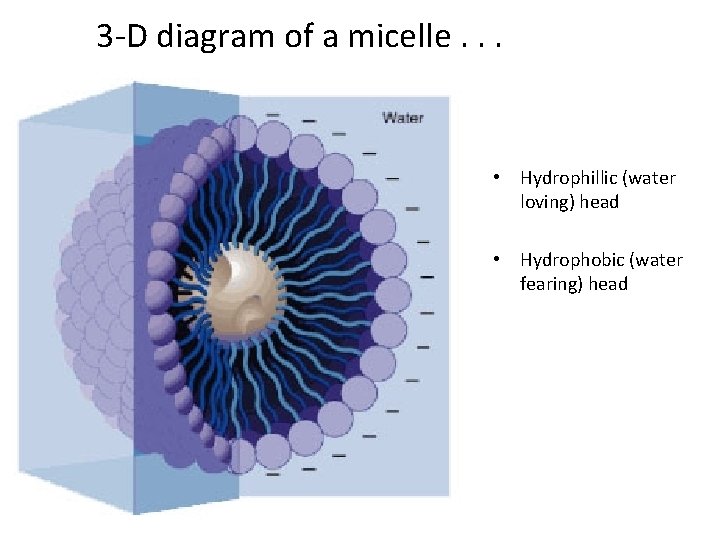
3 -D diagram of a micelle. . . • Hydrophillic (water loving) head • Hydrophobic (water fearing) head

“Hard” Water Reduces Efficiency of Soap • Hard water contains Ca 2+ and Mg 2+ ions • These cations react with the anionic portion of soap to form an insoluble “scum” –aka calcium stearate. • Can you think of a solution for better soap?

Predict, Observe, Explain • What will happen when food colouring is added to milk? What about when a drop of soap is added to the combination? Why? • http: //www. youtube. com/watch? v=LRZz 0 RAi u. Ho

Explanation • Milk is a mixture. It contains polar water, and nonpolar fat. • Food colouring is polar, so it doesn’t mix very well with the milk due to its fat content. • The soap is made of molecules that have two ends: a fat-friendly end a water-friendly end. • One end of soap attracts the fat in milk (the non-polar end), and the other end attracts the water in food coloring (the polar end). • The food coloring mixes with the milk because the dishwashing liquid acts as a bridge between the two.

How Did We Do? Learning Goal: I will be able to describe the properties of water that make it a good solvent for polar compounds I will explain the process of how soap works by using the terms hydrophobic and hydrophilic
- Slides: 19