Factors Affecting Solubility EXAM 2 REVIEW SESSION 20

Factors Affecting Solubility EXAM #2 REVIEW SESSION 20 th Date: Saturday, March Time: 1: 00 PM - 2: 30 PM Place: Here (Liquid N 2 ice cream with self-assembling syrup “caviar” will be served)
External Control of Solubility Temperature Pressure
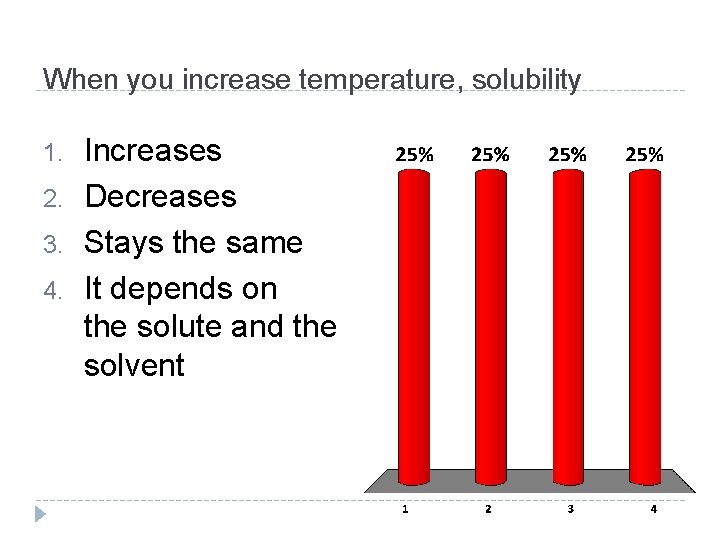
When you increase temperature, solubility 1. 2. 3. 4. Increases Decreases Stays the same It depends on the solute and the solvent
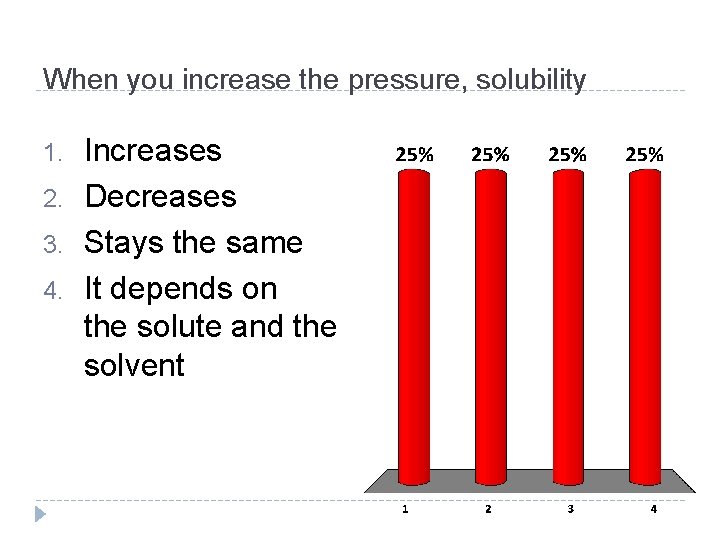
When you increase the pressure, solubility 1. 2. 3. 4. Increases Decreases Stays the same It depends on the solute and the solvent
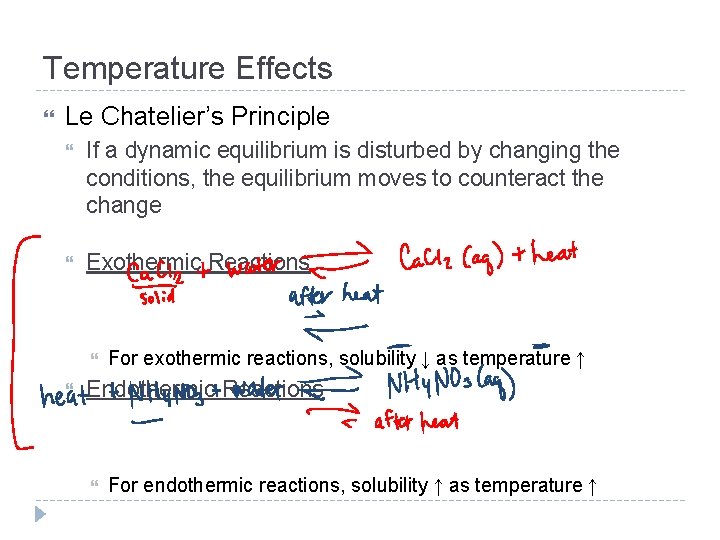
Temperature Effects Le Chatelier’s Principle If a dynamic equilibrium is disturbed by changing the conditions, the equilibrium moves to counteract the change Exothermic Reactions For exothermic reactions, solubility ↓ as temperature ↑ Endothermic Reactions For endothermic reactions, solubility ↑ as temperature ↑
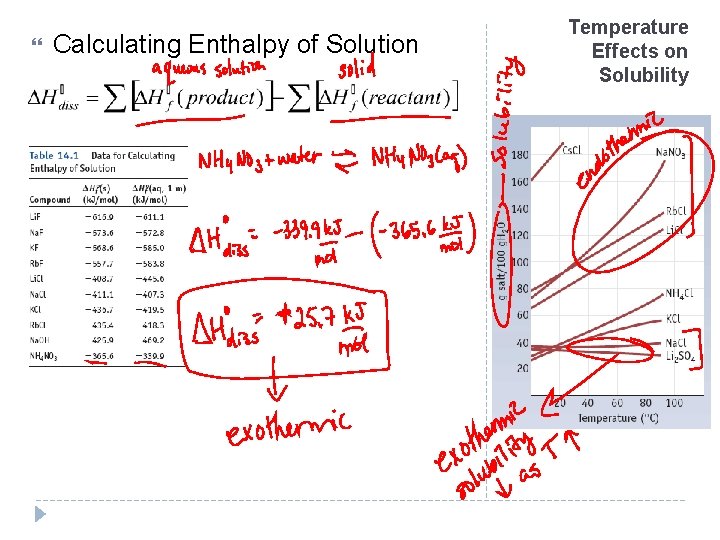
Calculating Enthalpy of Solution Temperature Effects on Solubility

Henry’s Law- Pressure and Gas Solubility

Colligative Properties What happens to the solvent when a solute is dissolved? Colligative properties Properties of the solvent that change upon dissolution Depend on number of solute molecules present
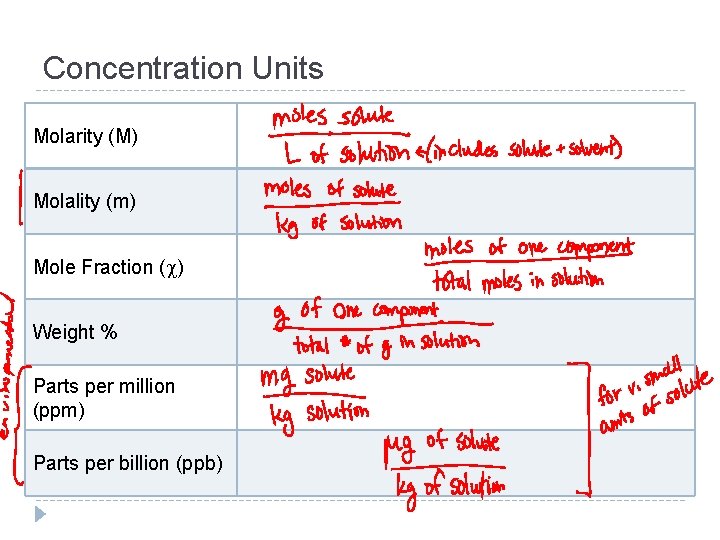
Concentration Units Molarity (M) Molality (m) Mole Fraction ( ) Weight % Parts per million (ppm) Parts per billion (ppb)
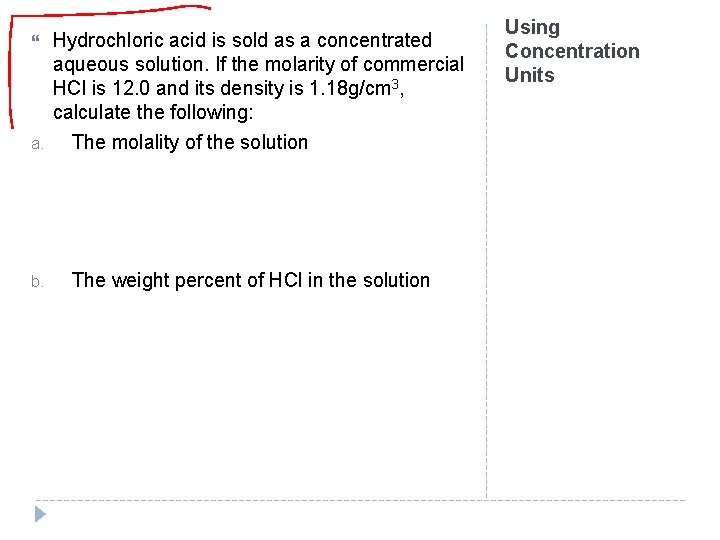
a. b. Hydrochloric acid is sold as a concentrated aqueous solution. If the molarity of commercial HCl is 12. 0 and its density is 1. 18 g/cm 3, calculate the following: The molality of the solution The weight percent of HCl in the solution Using Concentration Units

Colligative Properties What happens to the solvent when a solute is dissolved? Colligative properties= Properties of the solvent that change upon dissolution

Colligative Properties Raoult’s Law- Vapor Pressure Lowering Example: The vapor pressure of water at 20 o. C is 20. 1 mm Hg. What is the pressure of 100 g water mixed with 100 g ethylene glycol, C 2 H 2(OH)2?

Colligative Properties. Vapor Pressure Lowering Distillation: Changing the composition of a mixture of volatile liquids Vapor Pressures at 50 o. C Water=99 mm. Hg Ethanol=232 mm. Hg Distill a 10% alcohol solution.
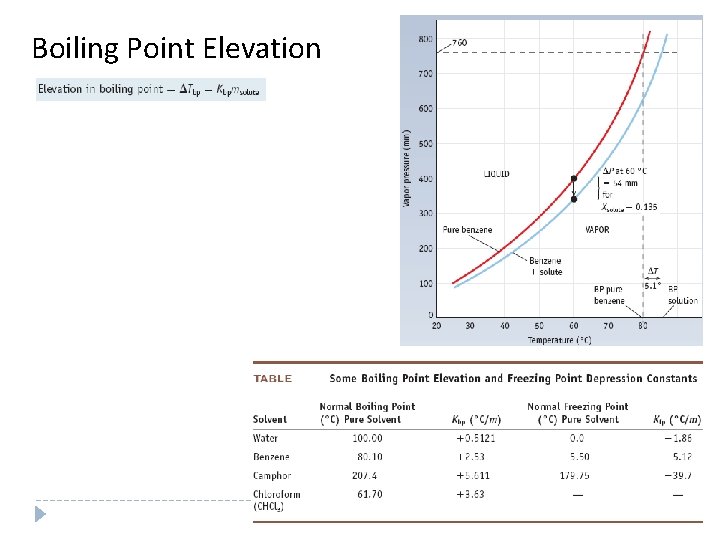
Boiling Point Elevation

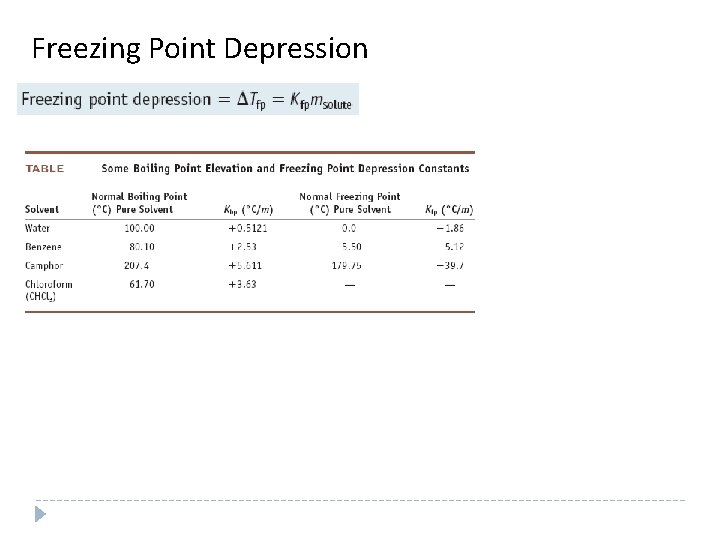
Freezing Point Depression

Effect of Ions
Which is better at melting ice, per gram, Na. Cl or Ca. Cl 2?
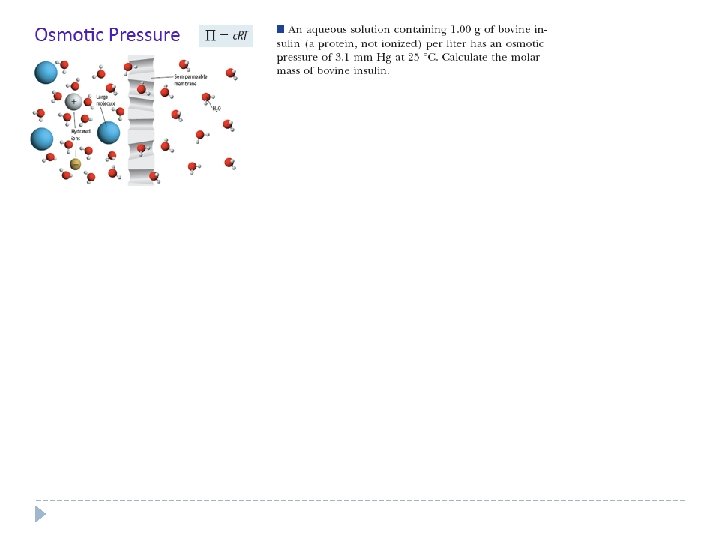
- Slides: 19