Factors Affecting Rate KINETICS4 Factors That Affect Reaction











- Slides: 32

Factors Affecting Rate KINETICS-4

Factors That Affect Reaction Rates 1. Nature of reactants Chemical reactivity How the reagents react to each other under specific conditions

Factors That Affect Reaction Rates 2. Physical State of the Reactants In order to react, molecules must come in contact with each other. The more homogeneous the mixture of reactants, the faster the molecules can react. Molecules or ions collide more easily in liquid solutions and gas phases. Heterogeneous reactions are affected by surface area

Factors That Affect Reaction Rates 3. Concentration of Reactants As the concentration of reactants increases, so does the likelihood that reactant molecules will collide.

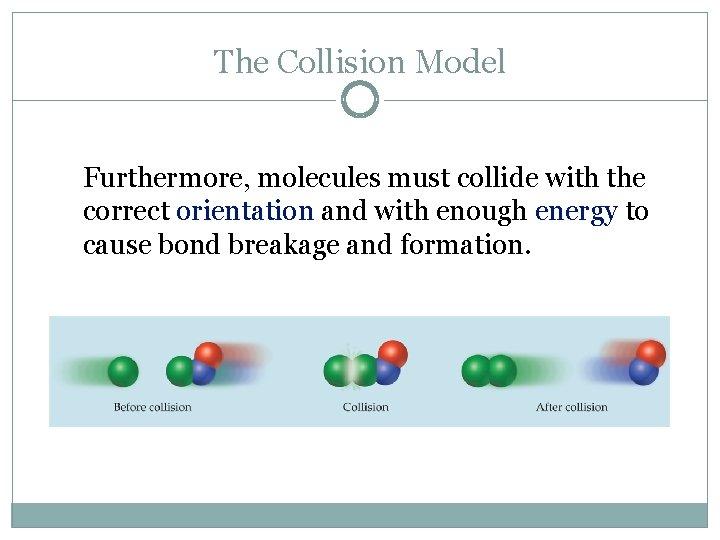
The Collision Model In a chemical reaction, bonds are broken and new bonds are formed. Molecules can only react if they collide with each other.

The Collision Model Furthermore, molecules must collide with the correct orientation and with enough energy to cause bond breakage and formation.

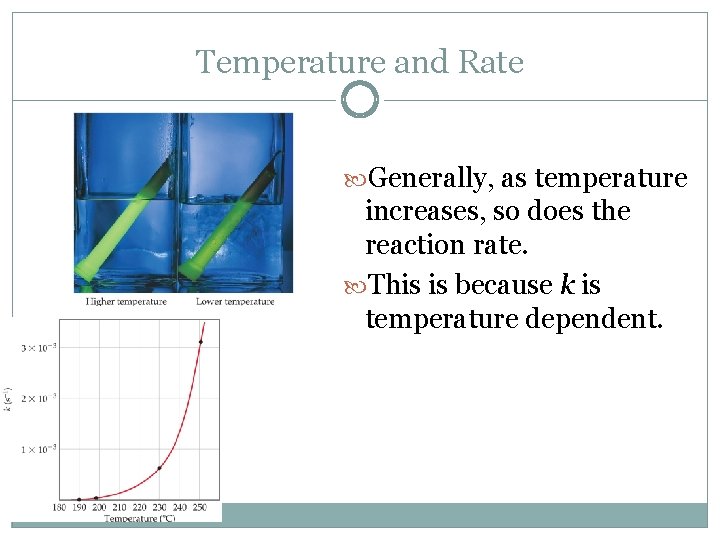
Factors That Affect Reaction Rates 4. Temperature At higher temperatures, reactant molecules have more kinetic energy, move faster, and collide more often and with greater energy. Generally an increase of 10˚C doubles the rate

Temperature and Rate Generally, as temperature increases, so does the reaction rate. This is because k is temperature dependent.

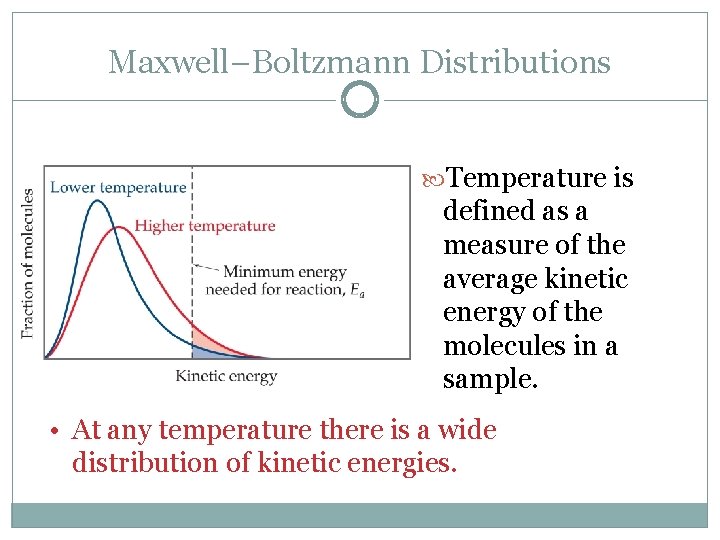
Maxwell–Boltzmann Distributions Temperature is defined as a measure of the average kinetic energy of the molecules in a sample. • At any temperature there is a wide distribution of kinetic energies.

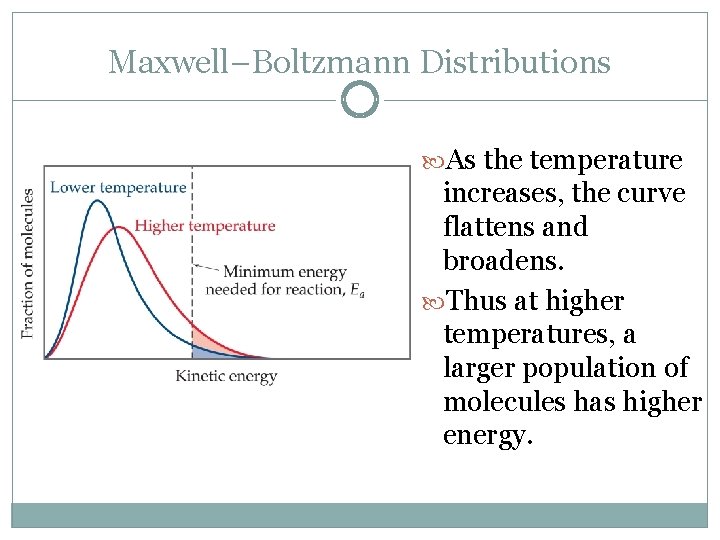
Maxwell–Boltzmann Distributions As the temperature increases, the curve flattens and broadens. Thus at higher temperatures, a larger population of molecules has higher energy.

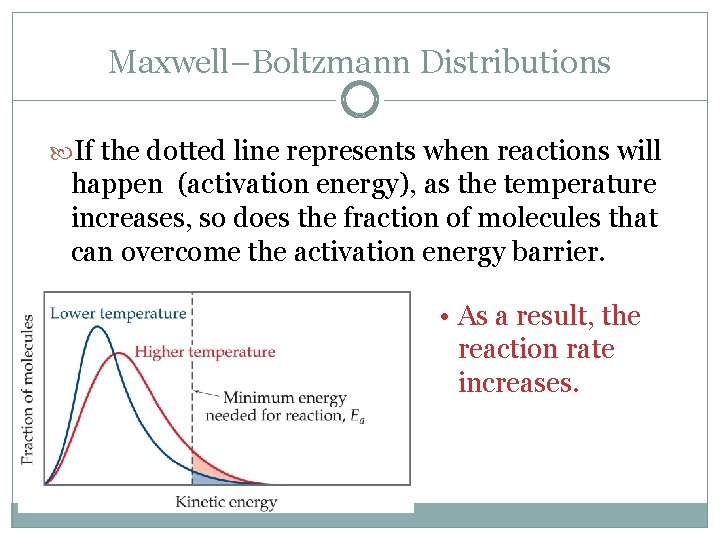
Maxwell–Boltzmann Distributions If the dotted line represents when reactions will happen (activation energy), as the temperature increases, so does the fraction of molecules that can overcome the activation energy barrier. • As a result, the reaction rate increases.

Factors That Affect Reaction Rates 5. Presence of a Catalyst Catalysts speed up reactions by changing the mechanism of the reaction. Catalysts are not consumed during the course of the reaction. Are not permanently consumed by reaction Homogeneous catalysts = I 2 clock reaction Heterogeneous catalysts = H 2 O 2 & KI

Activation Energy There is a minimum amount of energy required for reaction: the activation energy, Ea. Just as a ball cannot get over a hill if it does not roll up the hill with enough energy, a reaction cannot occur unless the molecules possess sufficient energy to get over the activation energy barrier.

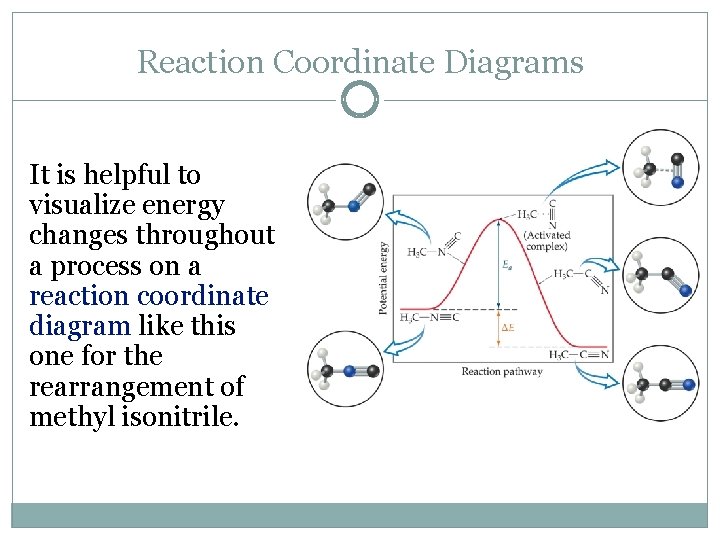
Reaction Coordinate Diagrams It is helpful to visualize energy changes throughout a process on a reaction coordinate diagram like this one for the rearrangement of methyl isonitrile.

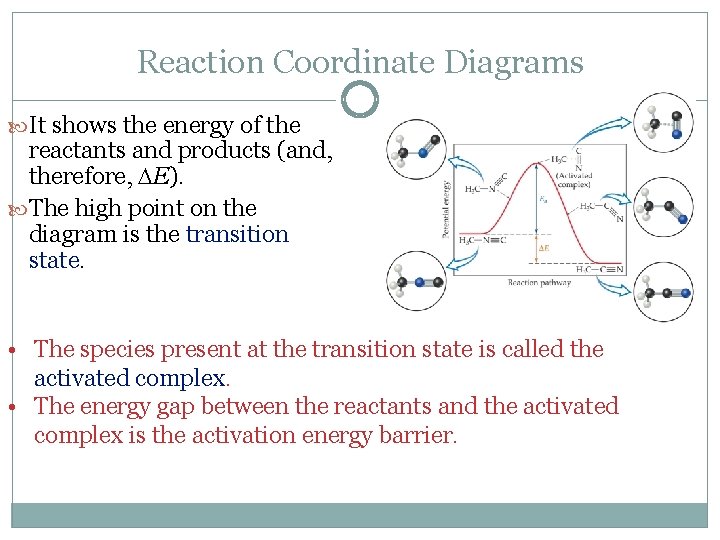
Reaction Coordinate Diagrams It shows the energy of the reactants and products (and, therefore, E). The high point on the diagram is the transition state. • The species present at the transition state is called the activated complex. • The energy gap between the reactants and the activated complex is the activation energy barrier.

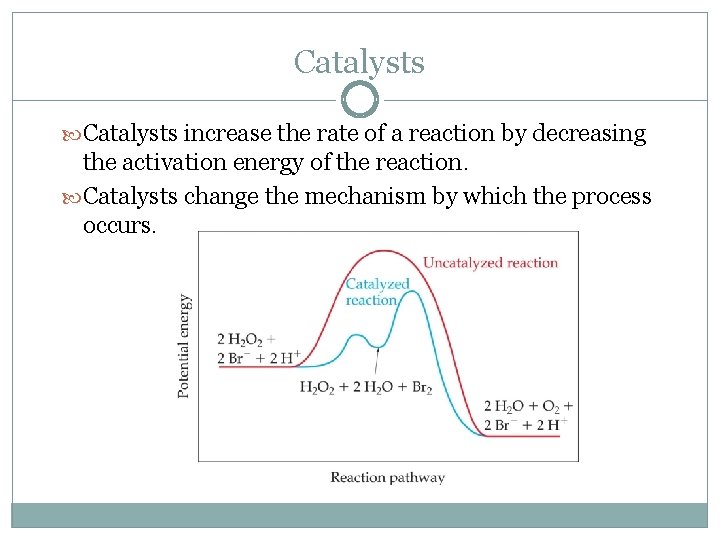
Catalysts increase the rate of a reaction by decreasing the activation energy of the reaction. Catalysts change the mechanism by which the process occurs.

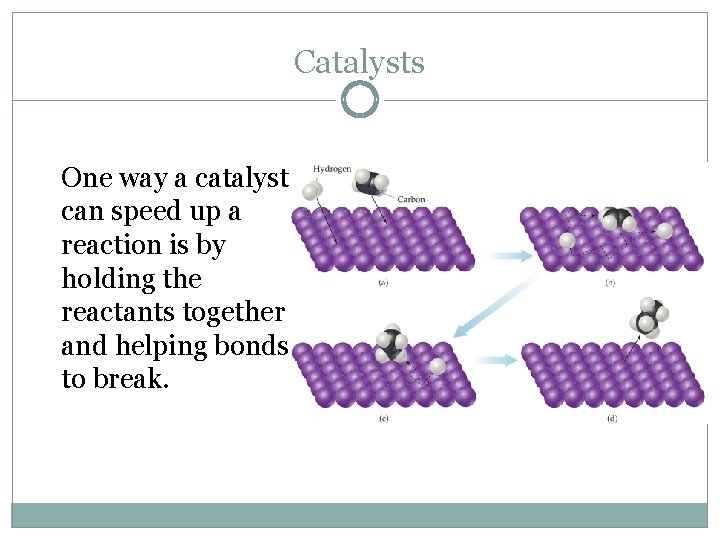
Catalysts One way a catalyst can speed up a reaction is by holding the reactants together and helping bonds to break.

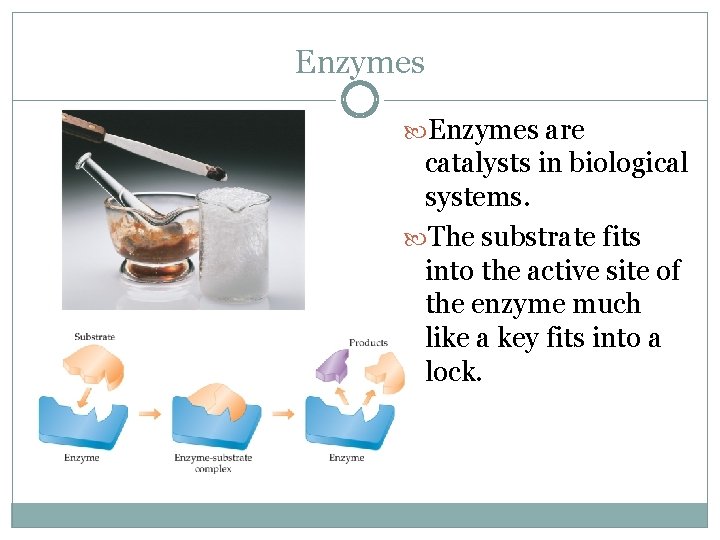
Enzymes are catalysts in biological systems. The substrate fits into the active site of the enzyme much like a key fits into a lock.

1. 2. 3. 4. Molecules react at rates proportional to their temperatures. Molecules all have the same average kinetic energy when they react. Molecules are moving very fast prior to reacting. Molecules must collide in order to react.

1. 2. 3. 4. Molecules react at rates proportional to their temperatures. Molecules all have the same average kinetic energy when they react. Molecules are moving very fast prior to reacting. Molecules must collide in order to react.

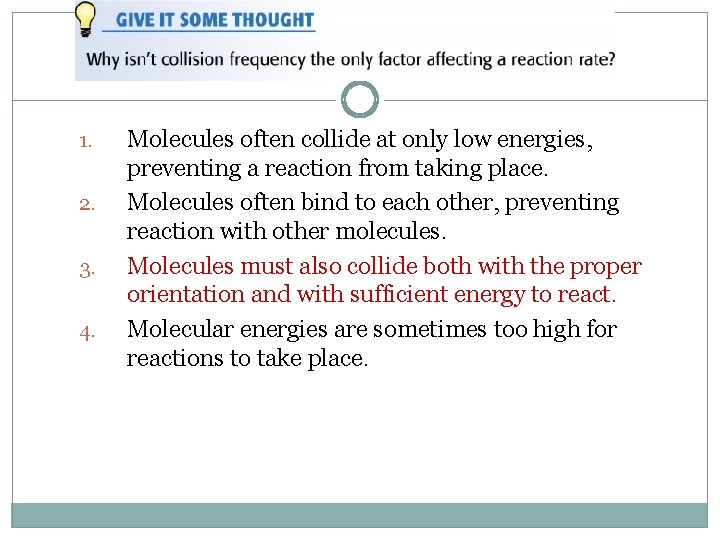
1. 2. 3. 4. Molecules often collide at only low energies, preventing a reaction from taking place. Molecules often bind to each other, preventing reaction with other molecules. Molecules must also collide both with the proper orientation and with sufficient energy to react. Molecular energies are sometimes too high for reactions to take place.

1. 2. 3. 4. Molecules often collide at only low energies, preventing a reaction from taking place. Molecules often bind to each other, preventing reaction with other molecules. Molecules must also collide both with the proper orientation and with sufficient energy to react. Molecular energies are sometimes too high for reactions to take place.

1. 2. 3. 4. bimolecular dimolecular termolecular unimolecular

1. 2. 3. 4. bimolecular dimolecular termolecular unimolecular

1. 2. 3. 4. All reactions are not elementary. Some information must be known about the rate constant to determine the rate law. Concentrations of reactant must be known to determine the rate law. The rate law depends not on the overall reaction, but on the slowest step in the mechanism.

1. 2. 3. 4. All reactions are not elementary. Some information must be known about the rate constant to determine the rate law. Concentrations of reactant must be known to determine the rate law. The rate law depends not on the overall reaction, but on the slowest step in the mechanism.

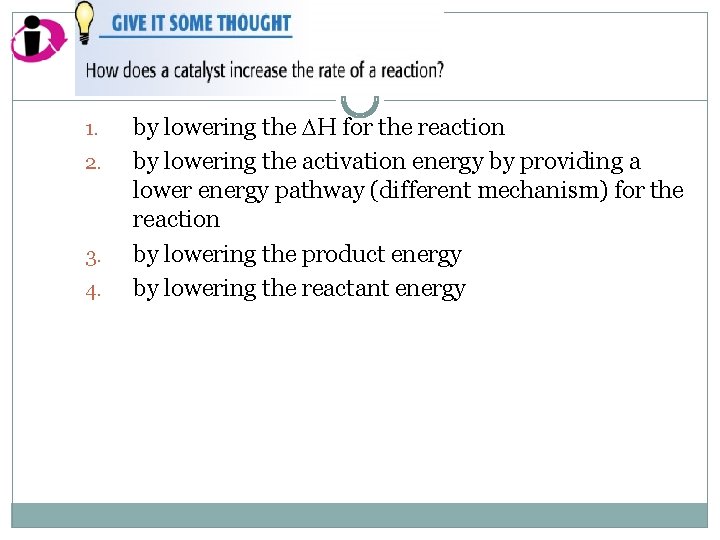
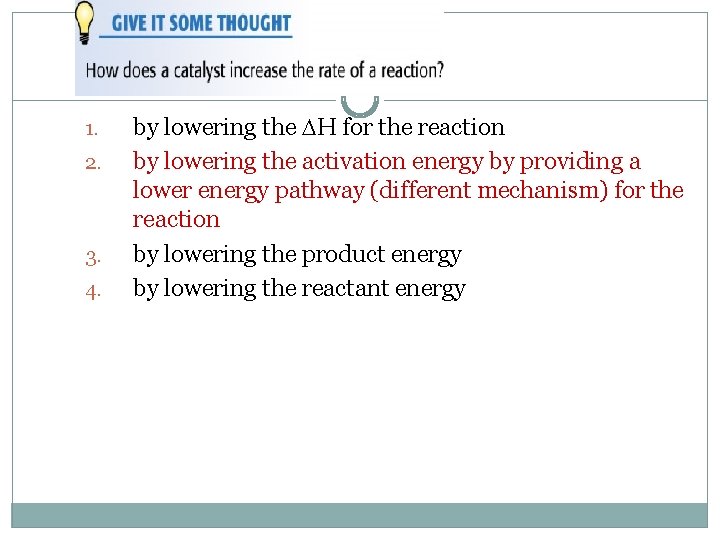
1. 2. 3. 4. by lowering the H for the reaction by lowering the activation energy by providing a lower energy pathway (different mechanism) for the reaction by lowering the product energy by lowering the reactant energy

1. 2. 3. 4. by lowering the H for the reaction by lowering the activation energy by providing a lower energy pathway (different mechanism) for the reaction by lowering the product energy by lowering the reactant energy

a. 1. 2. 3. 4. sweet spot hot spot active site binding site

a. 1. 2. 3. 4. sweet spot hot spot active site binding site

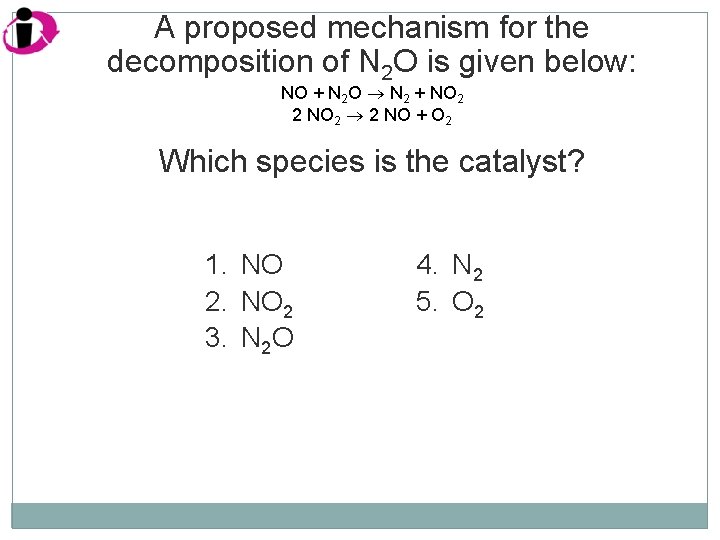
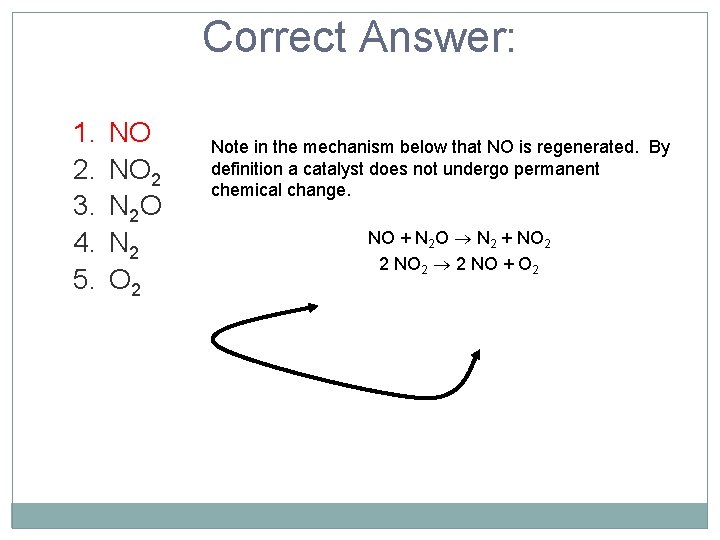
A proposed mechanism for the decomposition of N 2 O is given below: NO + N 2 O N 2 + NO 2 2 NO 2 2 NO + O 2 Which species is the catalyst? 1. NO 2 3. N 2 O 4. N 2 5. O 2

Correct Answer: 1. 2. 3. 4. 5. NO NO 2 N 2 O N 2 O 2 Note in the mechanism below that NO is regenerated. By definition a catalyst does not undergo permanent chemical change. NO + N 2 O N 2 + NO 2 2 NO 2 2 NO + O 2