FACTORS AFFECTING ABSORPTION OF DRUGS 1 FACTORS AFFECTING

FACTORS AFFECTING ABSORPTION OF DRUGS 1
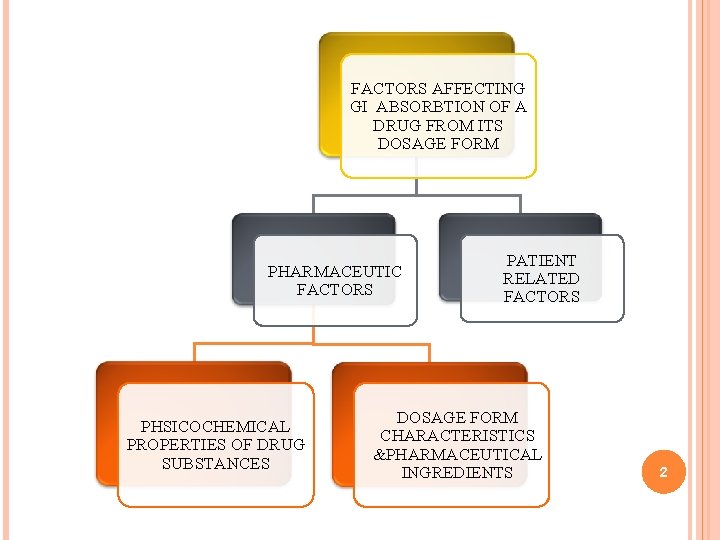
FACTORS AFFECTING GI ABSORBTION OF A DRUG FROM ITS DOSAGE FORM PHARMACEUTIC FACTORS PHSICOCHEMICAL PROPERTIES OF DRUG SUBSTANCES PATIENT RELATED FACTORS DOSAGE FORM CHARACTERISTICS &PHARMACEUTICAL INGREDIENTS 2
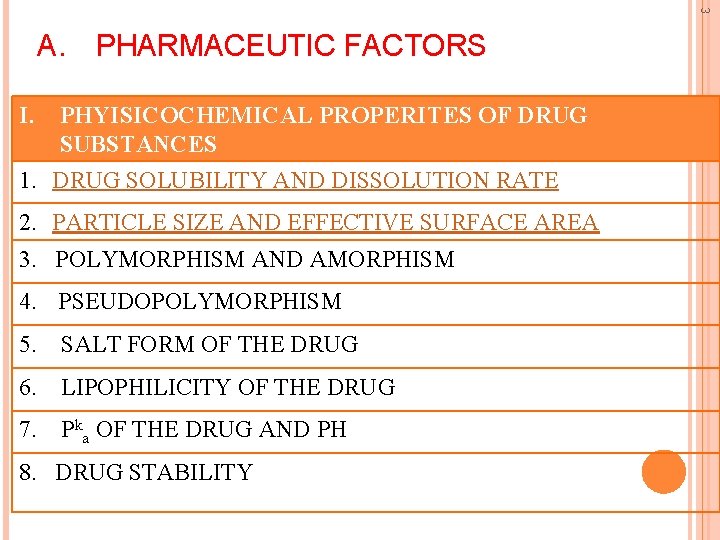
3 A. PHARMACEUTIC FACTORS I. PHYISICOCHEMICAL PROPERITES OF DRUG SUBSTANCES 1. DRUG SOLUBILITY AND DISSOLUTION RATE 2. PARTICLE SIZE AND EFFECTIVE SURFACE AREA 3. POLYMORPHISM AND AMORPHISM 4. PSEUDOPOLYMORPHISM 5. SALT FORM OF THE DRUG 6. LIPOPHILICITY OF THE DRUG 7. Pka OF THE DRUG AND PH 8. DRUG STABILITY
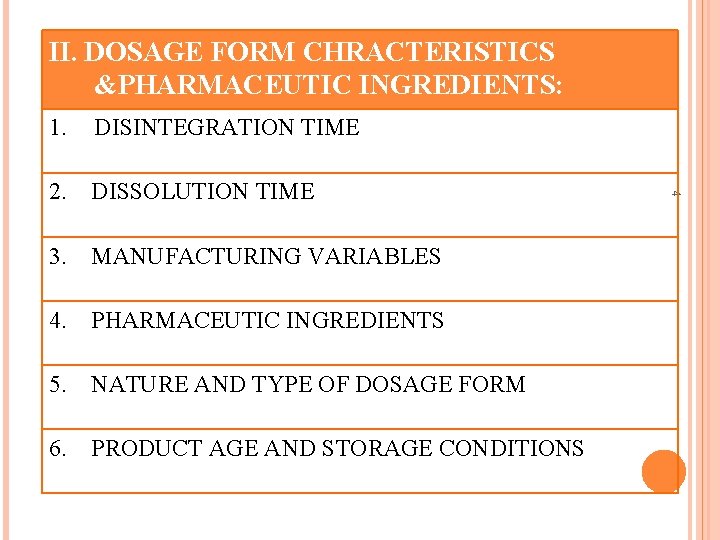
. II. DOSAGE FORM CHRACTERISTICS &PHARMACEUTIC INGREDIENTS: DISINTEGRATION TIME 2. DISSOLUTION TIME 3. MANUFACTURING VARIABLES 4. PHARMACEUTIC INGREDIENTS 5. NATURE AND TYPE OF DOSAGE FORM 6. PRODUCT AGE AND STORAGE CONDITIONS 4 1.
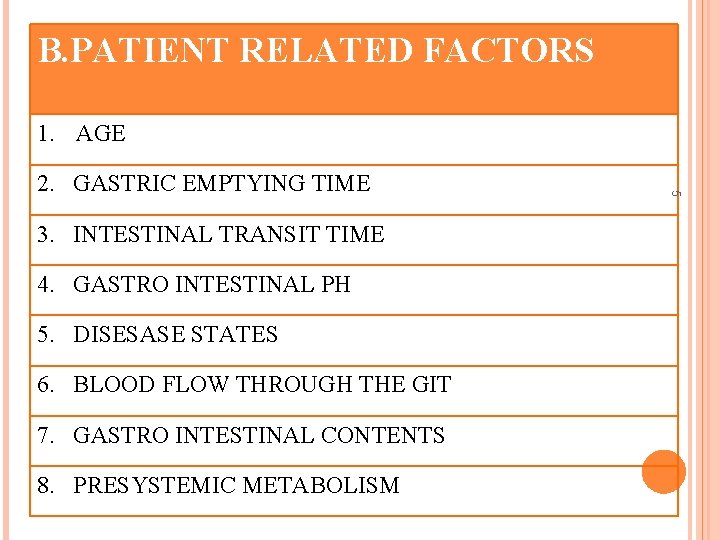
B. PATIENT RELATED FACTORS 1. AGE 3. INTESTINAL TRANSIT TIME 4. GASTRO INTESTINAL PH 5. DISESASE STATES 6. BLOOD FLOW THROUGH THE GIT 7. GASTRO INTESTINAL CONTENTS 8. PRESYSTEMIC METABOLISM 5 2. GASTRIC EMPTYING TIME

PHYSICOCHEMICAL PROPERTIES OF DRUG SUBSTANCES 6
1. Drug Solubility and Dissolution Rate : v For hydrophobic drugs i. e. poorly aqueous soluble drugs dissolution is the rate determining step (RDS). v Absorption of such drugs is called as dissolution rate limited. v Eg : Griseofulvin, Spironolactone. v For hydrophilic drugs i. e. drugs with high aqueous solubility permeation through biomembrane is the RDS. v Absorption of such drugs is called as permeation rate limited or trans membrane rate limited. v Eg: Cromolyn Sodium, Neomycin. 7
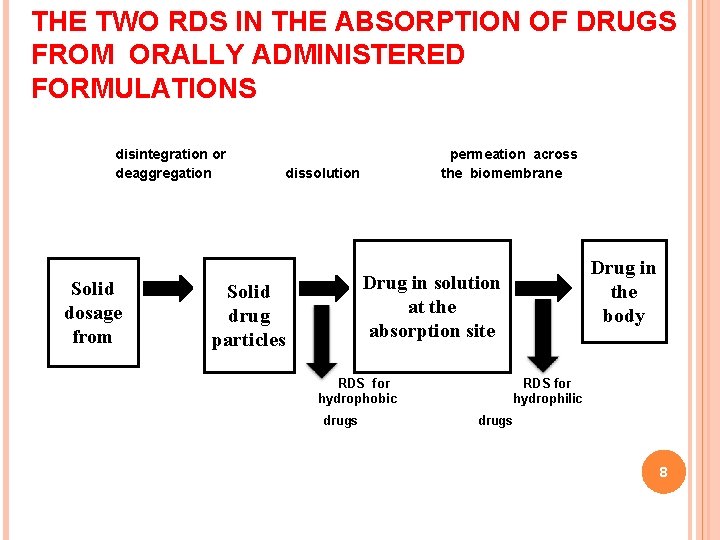
THE TWO RDS IN THE ABSORPTION OF DRUGS FROM ORALLY ADMINISTERED FORMULATIONS disintegration or deaggregation Solid dosage from permeation across the biomembrane dissolution Drug in the body Drug in solution at the absorption site Solid drug particles RDS for hydrophobic drugs RDS for hydrophilic drugs 8
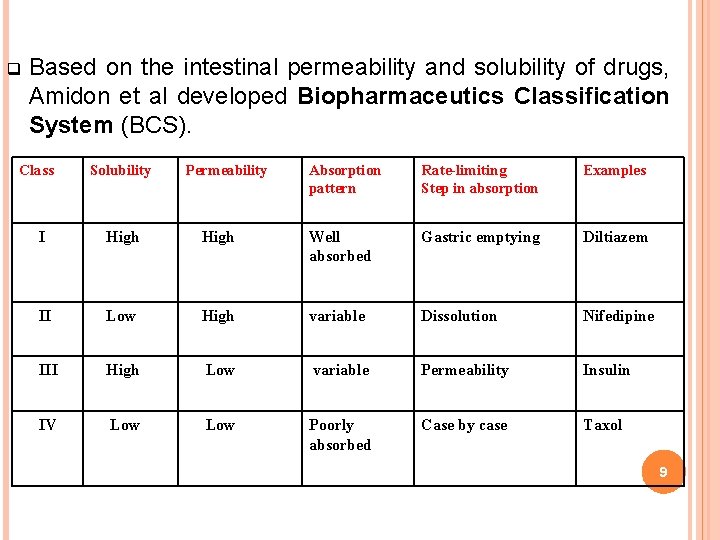
q Based on the intestinal permeability and solubility of drugs, Amidon et al developed Biopharmaceutics Classification System (BCS). Class Solubility Permeability Absorption pattern Rate-limiting Step in absorption Examples I High Well absorbed Gastric emptying Diltiazem II Low High variable Dissolution Nifedipine III High Low variable Permeability Insulin IV Low Poorly absorbed Case by case Taxol 9

DEFINITIONS Absolute/intrinsic solubility Defined as maximum amount of solute dissolved in a given solvent under standard condition of temperature pressure and p. H static property. Dissolution Process in which a solid substance solubilizes in a given solvent i. e. mass transfer from the solid surface to the liquid phase. 10
Rate of dissolution: The amount of drug substance that goes in solution per unit time under standardized conditions of temperature, p. H, constant solid surface area and solvent composition. Dynamic property. Ex: Cisaparide low solubility- but sufficient oral bioavailability. This because rapid dissolution rate despite low intrinsic solubility. 11
THEORIES OF DRUG DISSOLUTION q Diffusion layer model/Film theory. q Danckwert’s model/Penetration renewal theory. q Interfacial barrier model/Double barrier or Limited solvation theory. or surface 12
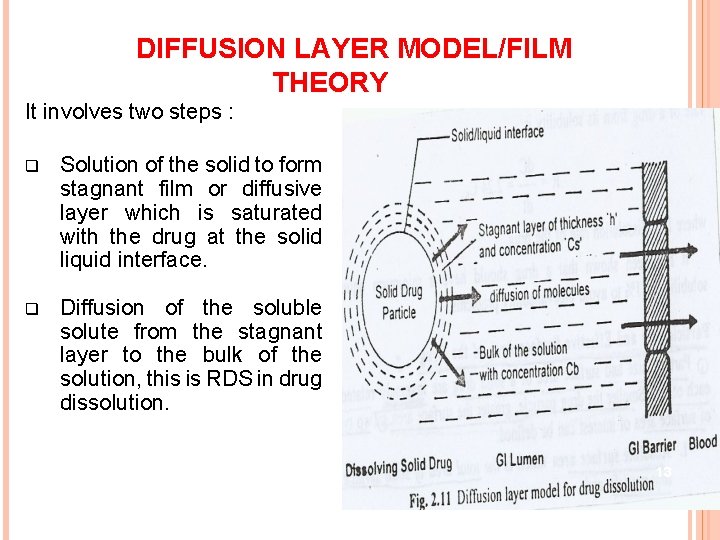
DIFFUSION LAYER MODEL/FILM THEORY It involves two steps : q Solution of the solid to form stagnant film or diffusive layer which is saturated with the drug at the solid liquid interface. q Diffusion of the soluble solute from the stagnant layer to the bulk of the solution, this is RDS in drug dissolution. 13
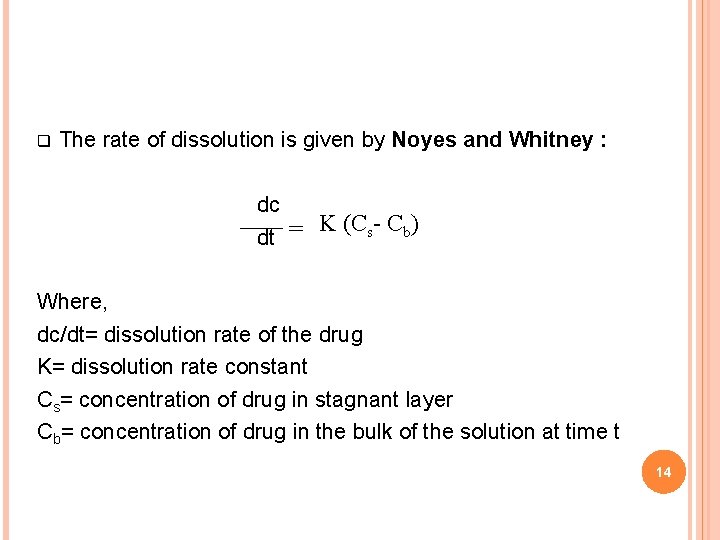
q The rate of dissolution is given by Noyes and Whitney : dc K (Cs- Cb) = dt Where, dc/dt= dissolution rate of the drug K= dissolution rate constant Cs= concentration of drug in stagnant layer Cb= concentration of drug in the bulk of the solution at time t 14
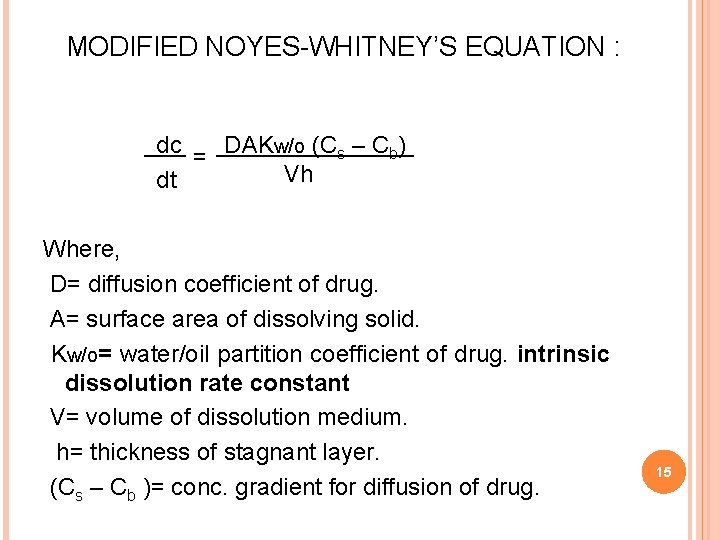
MODIFIED NOYES-WHITNEY’S EQUATION : dc = DAKw/o (Cs – Cb) Vh dt Where, D= diffusion coefficient of drug. A= surface area of dissolving solid. Kw/o= water/oil partition coefficient of drug. intrinsic dissolution rate constant V= volume of dissolution medium. h= thickness of stagnant layer. (Cs – Cb )= conc. gradient for diffusion of drug. 15
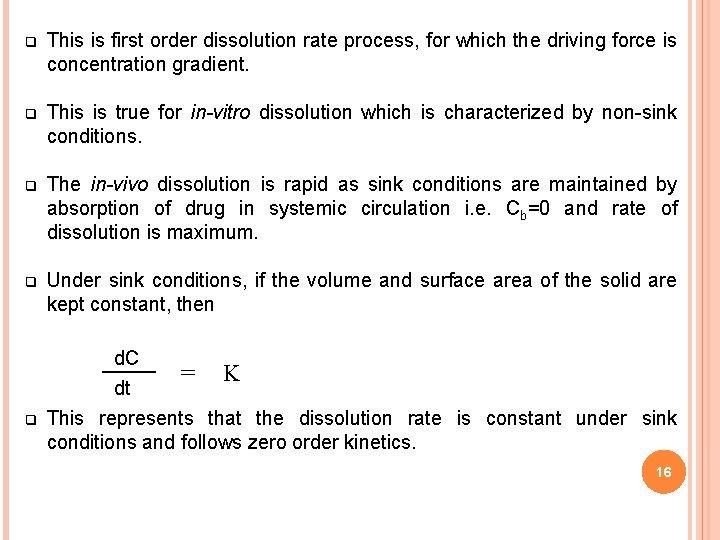
q This is first order dissolution rate process, for which the driving force is concentration gradient. q This is true for in-vitro dissolution which is characterized by non-sink conditions. q The in-vivo dissolution is rapid as sink conditions are maintained by absorption of drug in systemic circulation i. e. Cb=0 and rate of dissolution is maximum. q Under sink conditions, if the volume and surface area of the solid are kept constant, then q d. C = K dt This represents that the dissolution rate is constant under sink conditions and follows zero order kinetics. 16
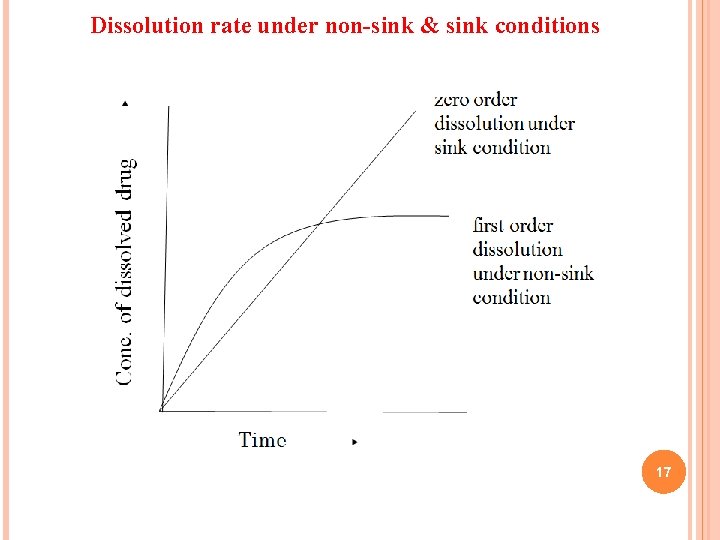
Dissolution rate under non-sink & sink conditions 17
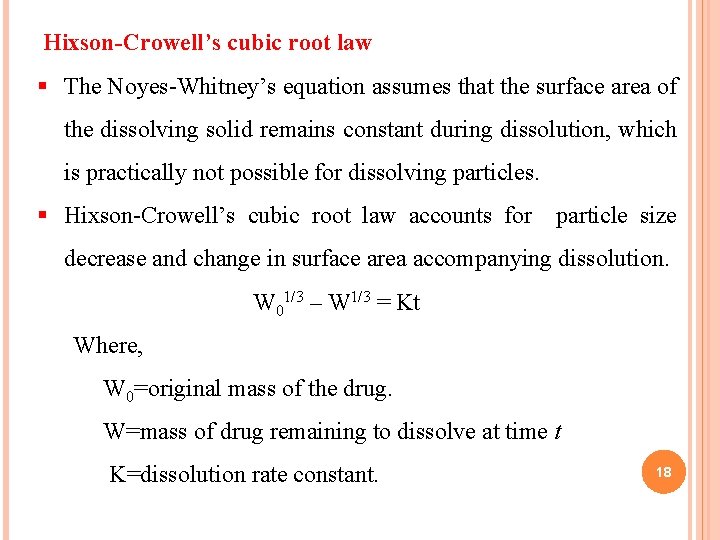
Hixson-Crowell’s cubic root law § The Noyes-Whitney’s equation assumes that the surface area of the dissolving solid remains constant during dissolution, which is practically not possible for dissolving particles. § Hixson-Crowell’s cubic root law accounts for particle size decrease and change in surface area accompanying dissolution. W 01/3 – W 1/3 = Kt Where, W 0=original mass of the drug. W=mass of drug remaining to dissolve at time t K=dissolution rate constant. 18
DANCKWERT’S MODEL / PENETRATION OR SURFACE RENEWAL THEORY v Danckwert’s did not approve the existence of stagnant layer because of liquid turbulence. v Takes into account the eddies or packets that are present in the agitated fluid which reach the solid-liquid interface, absorb the solute by diffusion and carry it into the bulk of solution. v These packets get continuously replaced by new ones and expose to new solid surface each time, thus theory is 19 called as surface renewal theory.
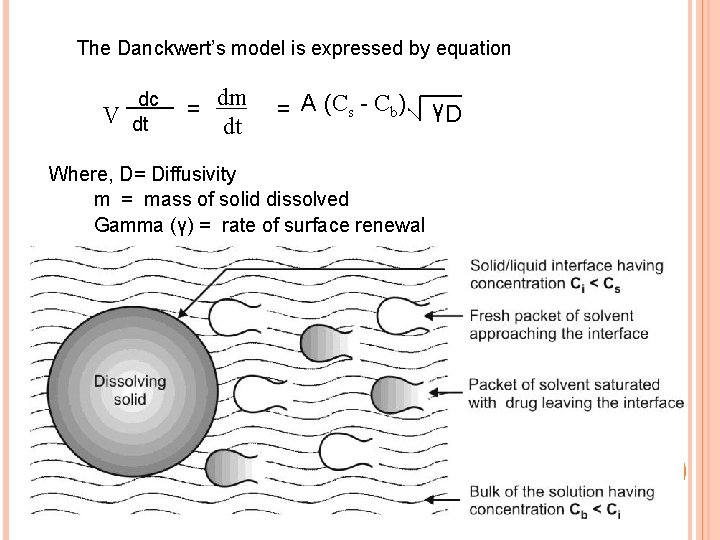
The Danckwert’s model is expressed by equation V dc dt = dm dt = A (Cs - Cb). γD Where, D= Diffusivity m = mass of solid dissolved Gamma (γ) = rate of surface renewal 20

Interfacial barrier model/Double barrier or limited solvation theory § Diffusion layer model and the Danckwert’s model were based on two assumptions: § The rate-determining step that controls dissolution is the mass transport. § Solid-solution equilibrium is achieved at the solid/liquid interface. § An intermediate concentration can exist at the interface as a result of solvation mechanism § As the activation energy required is more for solid therefore the rate of solubility of solid in the liquid becomes rate limiting rather then diffusion of dissolved solids 21

v The concept of this theory is explained by following equation G = Ki (Cs - Cb) G = dissolution rate per unit area Ki = effective interfacial transport constant. 22

Particle size & Effective surface area v Particle size and surface area of a solid drug are inversely related. v Two types of surface area ü ü v Absolute surface area Effective surface area Absolute surface area which is the total area of solid surface of any particle. v Effective surface area which is the area of solid surface exposed to the dissolution medium. 23
Hydrophilic Drugs v. Micronisation reduce particle size & surface of such particles have high energy than the bulk of the solid resulting increased interaction with the solvent of hydrophilic drugs results in ↑ dissolution rate in comparison to the simple milled form of these drugs. v. Micronisation v. Decreased dose, increased absorption efficiency Ex: Griseofulvin reduced to half and Spironolactone decreased 20 times 24
Hydrophobic drugs v. Micronisation of hydrophobic drugs (aspirin, phenacetin) decrease effective surface area of powders resulting fall in dissolution rate Reasons: v. Surface of the drug adsorbs air onto their surface which inhibits wettability. v. The particles re-aggregate to form larger particles due to their high surface free energy which either float on the surface or settle down 25
Overcome v. Use of surfactant as a wetting agent, decreases the interfacial tension & displaces the adsorbed air with the solvent. v. Polysorbate 80 increases the bioavailability of phenacetin v. Adding hydrophilic diluents such as PEG, PVP, Dextrose etc which coat the surface of hydrophobic drug particles and render them hydrophilic. 26
3. Polymorphism v Depending on the internal structure a solid can exist either in a crystalline or amorphous. v Substance exists in more than one crystalline form, the different forms are designated as polymorphs and the phenomenon as polymorphism Polymorphs are two types v Enantiotropic polymorph is the one which can be reversibly changed into another form by altering the temperature or pressure. ex: sulphur v Monotropic polymorph is the one which is unstable at all temperatures and pressures eg: glyceryl stearates. 27
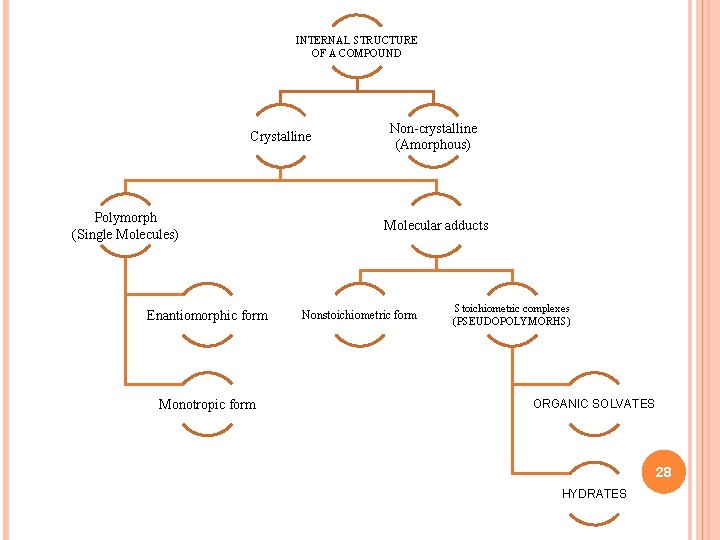
INTERNAL STRUCTURE OF A COMPOUND Crystalline Polymorph (Single Molecules) Enantiomorphic form Monotropic form Non-crystalline (Amorphous) Molecular adducts Nonstoichiometric form Stoichiometric complexes (PSEUDOPOLYMORHS) ORGANIC SOLVATES 28 HYDRATES
v Polymorphs differs in physical properties, solubility, Melting point, density, hardness and compression characteristics POLYMORPHISM v They are determined by techniques like optical crystallography, xray diffraction, differential scanning calorimetry etc. v One of the polymorphic forms will be physically more stable than others such a stable polymorphic represents the lowest energy state, has highest MP & least aqueous solubility. v other forms are metastable, low MP have greater solubility they show better availability and are preferred in formulation v Ex. chloramphenicol palmitate A, B, C, D. B shows bioavailability & A is virtually inactive biologically best 29
3. 1 Amorphous form v Drugs also exist as amorphous form have greater aqueous solubility than the crystalline forms energy required to transfer a molecule from lattice is greater than that required for amorphous v Ex: Cortisone acetate is 3 times more soluble than crystalline form v Order of dissolution is Amorphous>metastable>stable 30

4. Pseudopolymorphism v When the solvent molecules are incorporated in the crystal lattice of the solid - solvates v Solvates can exist in different crystalline form called as pseudopolymorphs. pseudopolymorphism. The phenomenon is called 4. 1. Hydrates and Solvates v In case entrapped Solvent is water then it is referred as Hydrate v Anhydrous form of the drug has greater aqueous solubility then the hydrates. (because already in interaction with water have less energy for crystal break up in comparison to the anhydrates). v Solvates differ in their physical parameters. 31

5. Salt form of the drug v Most of the drugs are either weak acids or weak bases. One of the easiest approaches to enhance the solubility and dissolution rate is convert them into their salt forms. v Weakly acidic drugs, a strong base salt is prepared such as the sodium and potassium salts of barbiturates and sulphonamides. v Weakly basic drugs a strong acid salt is prepared like the hydrochloride or sulphate salts of several alkaloidal drugs. v At a given p. H , the solubility of a drug, whether acidic/basic or its salt form is a constant. While considering the salt form of drug, p. H of the diffusion layer is imp not the p. H of the bulk of the solution 32
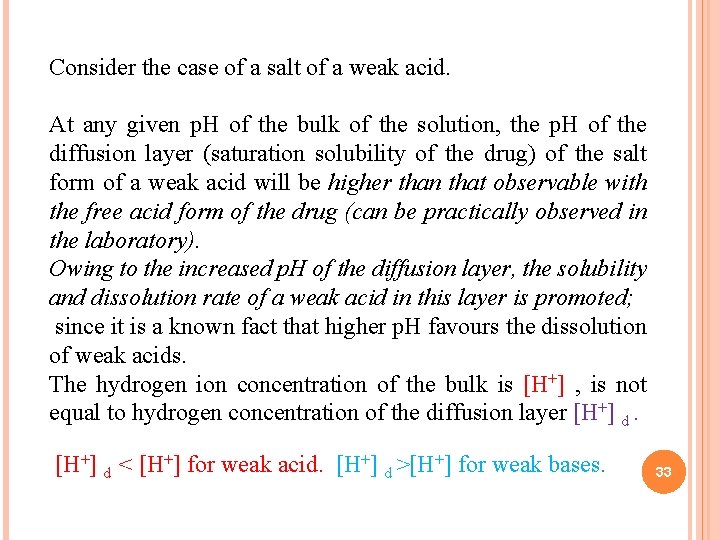
Consider the case of a salt of a weak acid. At any given p. H of the bulk of the solution, the p. H of the diffusion layer (saturation solubility of the drug) of the salt form of a weak acid will be higher than that observable with the free acid form of the drug (can be practically observed in the laboratory). Owing to the increased p. H of the diffusion layer, the solubility and dissolution rate of a weak acid in this layer is promoted; since it is a known fact that higher p. H favours the dissolution of weak acids. The hydrogen ion concentration of the bulk is [H+] , is not equal to hydrogen concentration of the diffusion layer [H+] d < [H+] for weak acid. [H+] d >[H+] for weak bases. 33

v Increases or decrease in the p. H of the diffusion layer is due the buffering action of strong cation or anion, which promotes ionization hence ↑ solubility and dissolution of weak acid or base. v When soluble ionic form moves into the bulk solution the p. H is lower or higher resulting in ppt of weak acid or base in form of fine particles which has increased surface area resulting in increased solubility 34
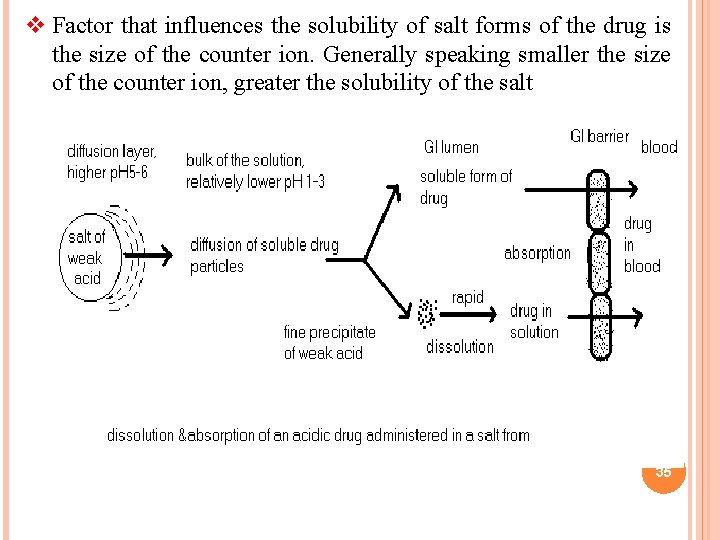
v Factor that influences the solubility of salt forms of the drug is the size of the counter ion. Generally speaking smaller the size of the counter ion, greater the solubility of the salt 35
The principle of in situ salt formation has been utilized to enhance the dissolution and absorption rate of certain drugs like aspirin and penicillin from buffered alkaline tablets. The approach is to increase the p. H of the microenvironment of the drug by incorporating buffer agents and promote dissolution rate. Apart from the enhanced bioavailability, buffered aspirin tablets have two more advantages: firstly, the gastric irritation and ulcerogenic tendency of the drug is greatly reduced, and secondly, the problem with the use of sodium salt of aspirin (to enhance the solubility) which otherwise has poor hydrolytic 36 stability, is overcome by in situ salt formation.
p. H PARTITION THEORY : The theory states that for drug compound of moleculer weight greater than 100, which are primarily transported across the biomembrane by passive diffusion , the process of absorption is governed by Dissociation constant p. Ka of drug. v Lipid solubility of the unionized drug (KO/W). v p. H of absorption site. v Hypothesis was based on the assumptions: v GIT is simple lipoidal barrier. v Larger the fraction of unionized drug, faster the absorption. v Greater the lipophilicity (KO/W) of unionized drug better the 37 absorption.
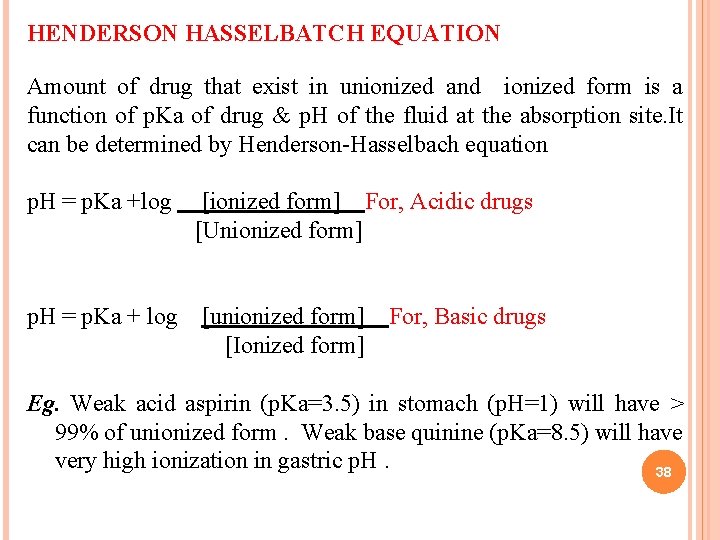
HENDERSON HASSELBATCH EQUATION Amount of drug that exist in unionized and ionized form is a function of p. Ka of drug & p. H of the fluid at the absorption site. It can be determined by Henderson-Hasselbach equation p. H = p. Ka +log [ionized form] For, Acidic drugs [Unionized form] p. H = p. Ka + log [unionized form] For, Basic drugs [Ionized form] Eg. Weak acid aspirin (p. Ka=3. 5) in stomach (p. H=1) will have > 99% of unionized form. Weak base quinine (p. Ka=8. 5) will have very high ionization in gastric p. H. 38
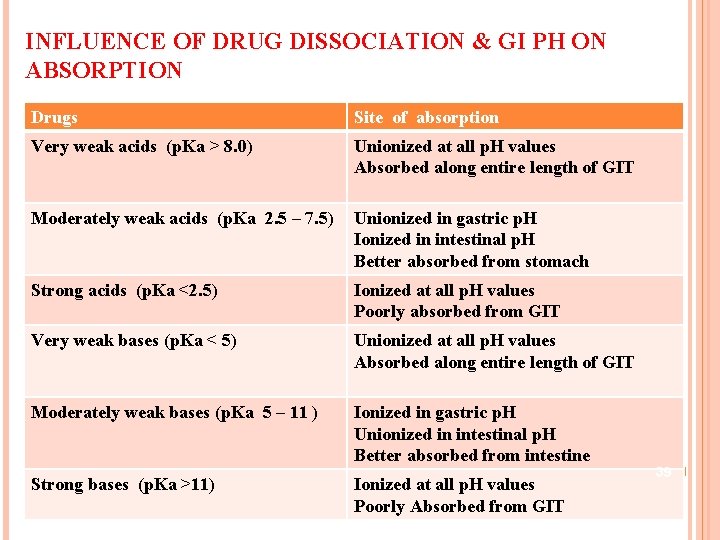
INFLUENCE OF DRUG DISSOCIATION & GI PH ON ABSORPTION Drugs Site of absorption Very weak acids (p. Ka > 8. 0) Unionized at all p. H values Absorbed along entire length of GIT Moderately weak acids (p. Ka 2. 5 – 7. 5) Unionized in gastric p. H Ionized in intestinal p. H Better absorbed from stomach Strong acids (p. Ka <2. 5) Ionized at all p. H values Poorly absorbed from GIT Very weak bases (p. Ka < 5) Unionized at all p. H values Absorbed along entire length of GIT Moderately weak bases (p. Ka 5 – 11 ) Ionized in gastric p. H Unionized in intestinal p. H Better absorbed from intestine Strong bases (p. Ka >11) Ionized at all p. H values Poorly Absorbed from GIT 39
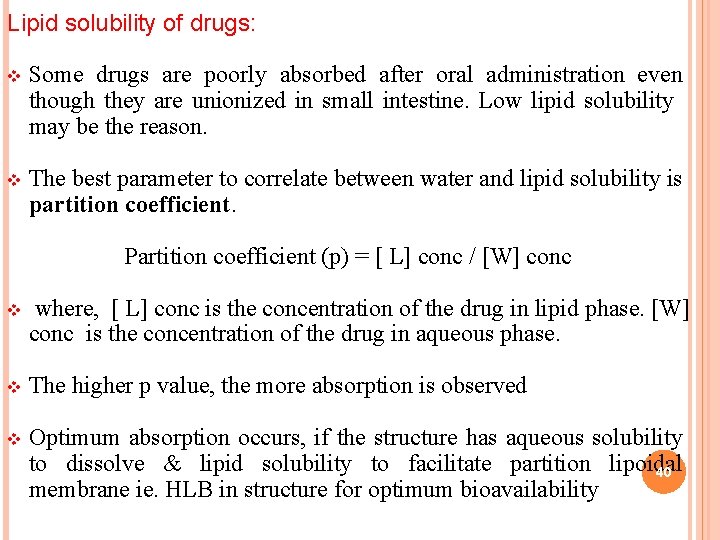
Lipid solubility of drugs: v Some drugs are poorly absorbed after oral administration even though they are unionized in small intestine. Low lipid solubility may be the reason. v The best parameter to correlate between water and lipid solubility is partition coefficient. Partition coefficient (p) = [ L] conc / [W] conc v where, [ L] conc is the concentration of the drug in lipid phase. [W] conc is the concentration of the drug in aqueous phase. v The higher p value, the more absorption is observed v Optimum absorption occurs, if the structure has aqueous solubility to dissolve & lipid solubility to facilitate partition lipoidal 40 membrane ie. HLB in structure for optimum bioavailability
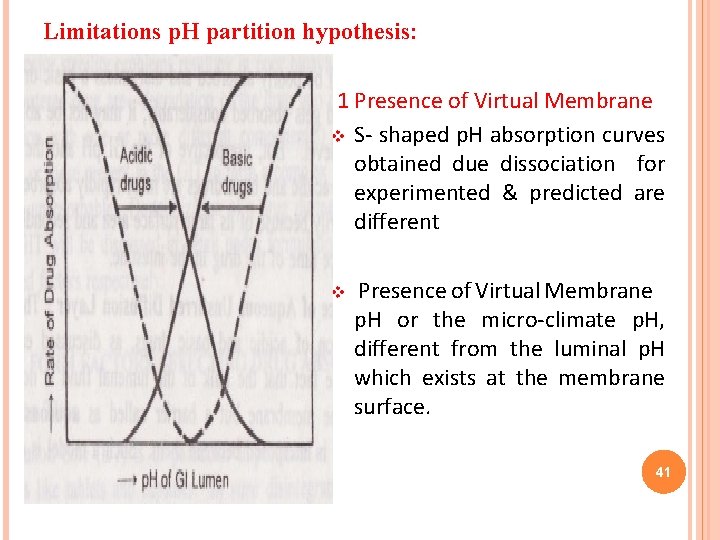
Limitations p. H partition hypothesis: 1 Presence of Virtual Membrane v S- shaped p. H absorption curves obtained due dissociation for experimented & predicted are different v Presence of Virtual Membrane p. H or the micro-climate p. H, different from the luminal p. H which exists at the membrane surface. 41
2. Absorption of the ionized drug v Despite of the assumption that only un-ionized and lipophilic drugs are absorbed to a greater extent, some drugs e. g. Morphinan derivatives which are much more ionized are absorbed passively v Structure has very high lipophilicity. 3. Influence of the GI surface area and residence time of the drug: v Irrespective of the GI p. H and degree of ionization, both acidic and basic drugs are more rapidly absorbed from the intestine. v Primarily because of its large surface area and secondly, because 42 of the long residence time of the drug in the intestine.
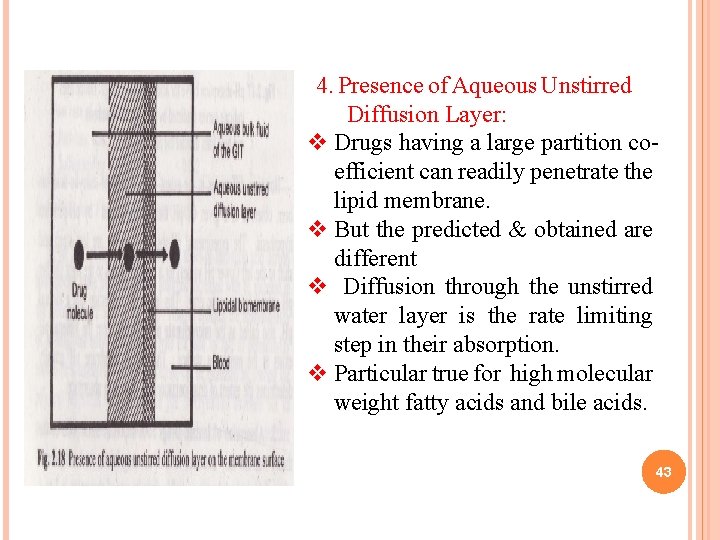
4. Presence of Aqueous Unstirred Diffusion Layer: v Drugs having a large partition coefficient can readily penetrate the lipid membrane. v But the predicted & obtained are different v Diffusion through the unstirred water layer is the rate limiting step in their absorption. v Particular true for high molecular weight fatty acids and bile acids. 43
8. Drug stability v A drug for oral use may destabilize either during its shelf-life or in the GIT resulting in poor bioavailability. v Degradation of drug into inactive form by interaction with one or more different component(s) to form a complex that is poorly soluble or is un-absorbable 9. Stereochemical nature of drug: v Enantiomers possess identical physical and chemical properties despite significant differences in spatial configuration. v Stereoselectivity is not expected effect during passive absorption of enatiomers. 44

v This is not generally true if an active or receptor-mediated process is involved. v Absorption may exhibit stereo selectivity , giving rise to the possibility of competitive interaction between enatiomers. v Ex. intestinal absorption of D-cefalexin via dipeptide transport system can be inhibited by its L-enatiomer. 45

DOSAGE FORM CHARACTERISTICS AND PHARMACEUTIC INGREDIENTS 46
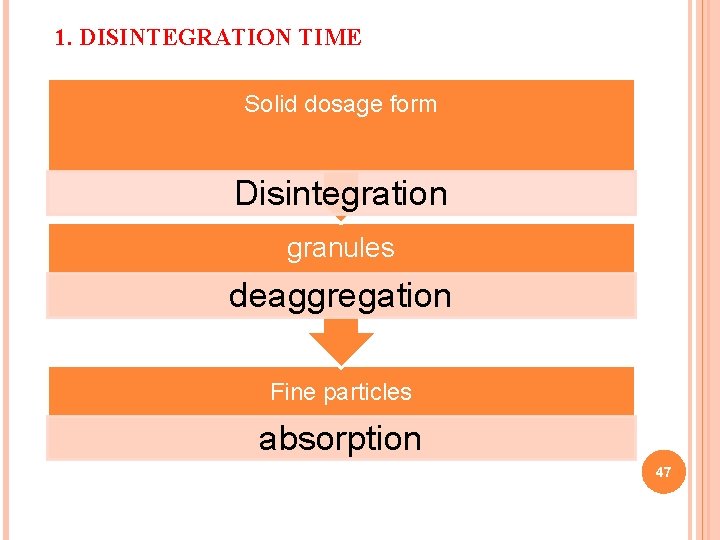
1. DISINTEGRATION TIME Solid dosage form Disintegration granules deaggregation Fine particles absorption 47

v Solid dosage form should conform to disintegration time, otherwise leads to slower dissolution resulting in insufficient the absorption resulting in bioavailability problems v Rapid disintegration is important in therapeutic success of solid dosage form. v Disintegration time of the tablet is directly related to the amount of binder present and compressional force of the tablet. v Disintegration can be aided by incorporating disintegrants in suitable amounts during formulation. v Dissolution in such fine particles is faster than that from granules.
2. MANUFACTURING VARIABLES Solid dosage form related factors that influence dissolution: v Excipients v Manufacturing processes v Tablet : method of granulation , compression force v Capsule: intensity of powder packing Method of granulation Wet granulation Limitations I. Formation of crystal bridge by the presence of liquid II. Liquid may cause process like hydrolysis III. Dry step may effect thermolabile substances IV. Method & duration of blending V. Method, time & temperature of drying
Direct compression v Agglomerative phase of communition v Involves grinding of drugs in a ball mill for time long enough to affect spontaneous agglomeration. v Tablet produced will be stronger and show rapid dissolution v Attributed- increase in internal surface area Compression force(CF) v CF employed in tableting influences density, porosity, hardness DT and dissolution of tablets. v Higher CF increases density & hardness of tablet, decreases porosity and hence↓ penetrability

v Retards wettability by forming a firmer and more effective sealing layer by the lubricant v Promotes tighter bonding between the particles v Result in slowing of the dissolution rate of tablets v Higher CF cause deformation, crushing or fracture of drug particles into smaller ones with a large increase in the effective surface, results in increased in dissolution rate 51
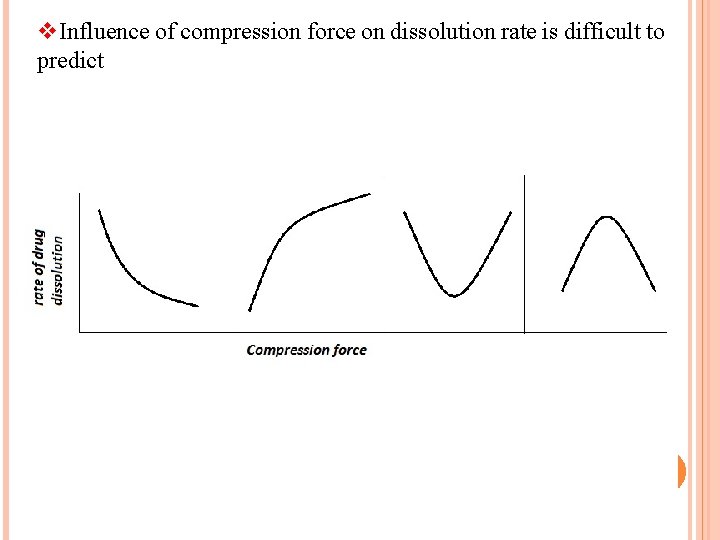
v. Influence of compression force on dissolution rate is difficult to predict 52
Intensity of packing of capsule contents v Packing density of capsule content can either inhibit or promote dissolution v Diffusion of GI fluids into the tightly filled capsules creates a high pressure within the capsule resulting in rapid bursting & dissolution v Particles having fine size & hydrophobic in nature result in tightly packed mass with decrease porosity & penetrability z
Excipients/Pharmaceutic Ingredients: v Substances which are added to ensure acceptability, physicochemical stability during the shelf life , uniformity of composition optimum bioavilability and function-ability of the drug product. v Despite their inertness and utility in the dosage form, excipients can influence the absorption of the drug 54

1. Vehicle v Major component of liquid orals & Parenterals. v Aqueous vehicles, Nonaqueous water miscible-glycerol, Nonaqueous water immiscible vehicles-vegetable oils. v Bioavailability of a drug depends to a large extent on its miscibility with the biological fluids v Aqueous vehicles are miscible with body fluid helping in rapid absorption v Water immiscible vehicles absorption depends on its partitioning from oil phase to aqueous body fluids- slower v Viscosity of the vehicle influence diffusion
2. Diluents/fillers v Added to increase bulkiness may be organic or inorganic v Hydrophilic powders promote dissolution of poorly water soluble drugs(steroids) by forming a coat rendering them hydrophilic. v Drug diluent interaction can result in poor bioavilability: DCP-Tetra 56
3. Binders and granulating agents v Materials used to hold powders together by promoting cohesiveness. Binders used are polymeric materials eithere natural, semisynthetic and synthetic like starch, cellulose , PVP, etc. v Hydrophilic binders promote dissolution of by imparting hydrophilic properties to granules surface. v Large amount of binder increases hardness & decrease dissolution & disintegration rates of tablets. v PEG 6000 forms complex with phenobarbital ↓ solubility v Nonaqueous binders like ethylcellulose retard Disso 57 v
v 4. Disintegrants v Agents that break cohesive strength of tablet v A decrease in amount of disintegrant can significantly lower bioavilability. v They are hydrophilic in nature v Adsorbing disintegrants bentonite , veegum , adsorb the low dose drug ↓ absorption v Microcrystalline cellulose at high CF retard Disso

5. Lubricants Aids flow of granules & reduce interparticulate friction. v Hydrophobic in nature (metallic stearates and waxes) v known to inhibit wettability , penetration of water into tablet and their dissolution/disintegration v It can be overcome by adding them just before compression v Use soluble lubricants like carbowaxes & SLS 59 v

6. Coatings v Effect of various coating on dissolution: Enteric coating>sugar coating>non enteric film coating v Dissolution profile of coating may change on ageing. Ex: shellac coated tablets on long storage show slows dissolution. v Overcome by incorporating PVP 60
7. Suspending agents v Reduce the settling of particles by increasing viscosity of the continuous phase v Macromolecular gums form unabsorbable complex: CMCAmphetamine v Viscosity enhancers act as a mechanical barrier to the diffusion of the drug from dosage form into GI fluids v Form a viscid layer on the mucosa v They also retard the GI transit of the drug.
8. Surfactants v Used as wetting agents , Solubilizers, emulsifiers v Increasing absorption Ø Promotion of wetting by increasing effective surface area Ø Better membrane contact of drug Ø Enhance membrane permeability of drug v Decreasing absorption Ø Unabsorbable drug micelle complex formation at conc. greater CMC Ø Laxative action –large surfactant concentration v Synthetic surfactants show their action at pre CMC. But natural surfactants at CMC

9. Buffers v Create a right atmosphere for drug dissolution(buffered aspirin tablets) v Certain buffers containing potassium ions inhibits the absorption(vit. B 2, sulfanamide)r by decresing effective drug concentrarion v Overcome buffer system should contain same cation as the drugsalt.
10. Complexing agents v Enchance bioavilability Ø highly soluble complex : Ergotamine tartarate-caffeine Ø ↑ lipophilicity: caffeine-PABA complex membrane Ø ↑ permeability by chelation membrane ions -GI absorption of heparin in presence of EDTA. v Decreased bioavailability Ø Large size complex Ø Failure of the complex to dissociate

11. Colourants Water soluble dye have inhibitory effect on dissolution rate of several crystalline drugs. v Dye get adsorbed onto the crystal phase ↓ESA retards dissolution-sulfathiazole v Inhibit miceller solubilization v Cation dyes more reactive anion dyes(>adsorption on primary particles) 65 v ex : brittle blue

12. Crystal growth inhibitors: v Solution with high dissolved conc. of drug precipitation or crystal formation tend show v Prevent crystal growth or ppt. Eg. PVP , PVG v Increasing the viscosity vehicle v Inhibit conversion of high energy metastable polymorph to stable, less soluble polymorph v Adsorbing on crystal surface preventing crystal growth 66
NATURE AND TYPE OF DOSAGE FORM v Two to five fold difference in oral bioavailability occurs for a drug depending on nature & type of dosage form v This is due to variation in release rate from the dosage v Complicated the dosage form greater the rate limiting steps & bioavailability variation v Bioavilability of the drug from various dosage form decreases in following order: v Solutions>emulsions>suspensions>capsules>tablets>coatedtablets> enteric coated tablets>sustained release products.
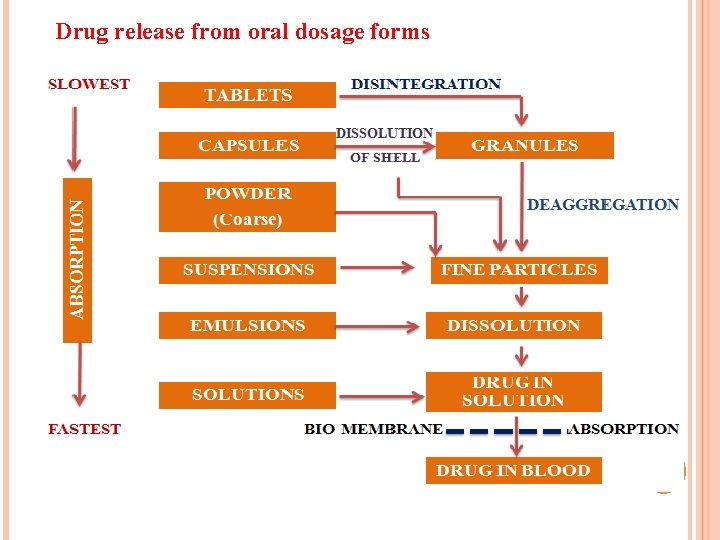
Drug release from oral dosage forms 68

Solutions Factors-nature of solvent , viscosity , surfactants, solubilizers, stabilizers, etc. v Dilution may result in precipitation sometimes. v Factors that limit drug formulation in solution are solubility, cost etc. 69 v stability,

Emulsions v They present a large surface area of oil to the GIT for absorption of a drug v Drug administered in oily vehicle emulsified are solubilized in the GIT by bile salts forming mixed micelles v Drug absorbed directly into the lymphatic system avoiding first-pass metabolism v Ex: Indoxole dispensed as emulsion increases absorption 3 fold over its aqueous suspension 70

Suspension v Rate limiting step in absorption of a drug is drug dissolution, due to large surface area of the particles v Particle size, polymorphism, suspending agents wetting agents, viscosity, Powders v Particle size, polymorphism, wetting etc. v. Drug formulation in powders is limited due to handling and palatability problems.

Capsules v Powders and granules are administered in hard capsules v Particle size, polymorphism, density, intensity of packing and nature of diluents v Hydrophobic drugs of fine particle size tightly pack resulting in decreased porosity with decreased penetrability v Incorporating a large amount of hydrophilic diluents (upto 50%), a small amount of wetting agent as SLS ( upto 1%) counters the said problem

v Interaction between the drug and the diluents (eg: - tetracycline. DCP) or gelatin shell. v Viscous fluids and oils are packed in soft capsules v Dissolves faster and show better drug availability than hard caps v High water content in shell (above 20%) migrates into capsule & crystallization occurs during the drying stage resulting in altered drug dissolution characteristics. 73

Tablets v Bioavailability problem may arise due to reduction in surface area due to granulation & compression v Processing & physicochemical properties of drug & diluents Coated tablets v Coating acts as a barrier which must first dissolve to give way to dissolution and disintegration of tablets v Sugar coat takes longer time to dissolve than film coat v Seal coat on aging effect DT

Enteric coated tablets v Bioavailability problems occur as coat dissolves in intestinal p. H which takes 2 to 4 hours v This variability in gastric emptying depends on nature of meal & GI motility v Thickness & aging of shellac enteric coat Sustained release products v Drug release is most unpredictable v Problems from dose dumping and no drug release.
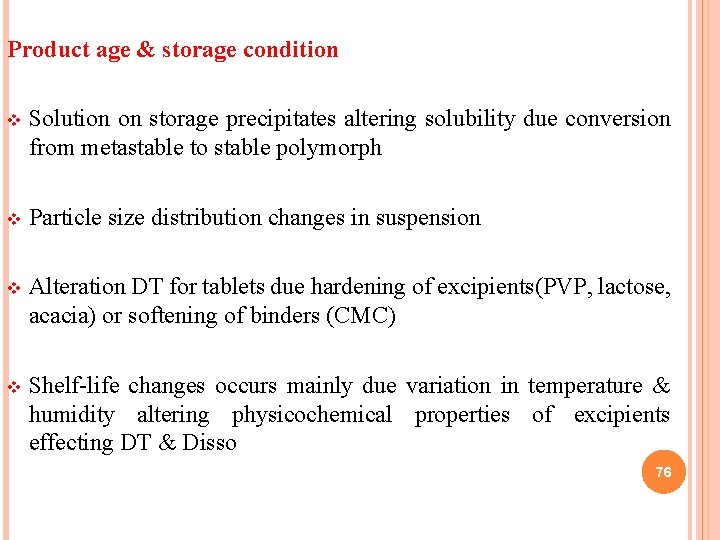
Product age & storage condition v Solution on storage precipitates altering solubility due conversion from metastable to stable polymorph v Particle size distribution changes in suspension v Alteration DT for tablets due hardening of excipients(PVP, lactose, acacia) or softening of binders (CMC) v Shelf-life changes occurs mainly due variation in temperature & humidity altering physicochemical properties of excipients effecting DT & Disso 76

PATIENT RELATED FACTORS AFFECTING DRUG ABSORPTION 77
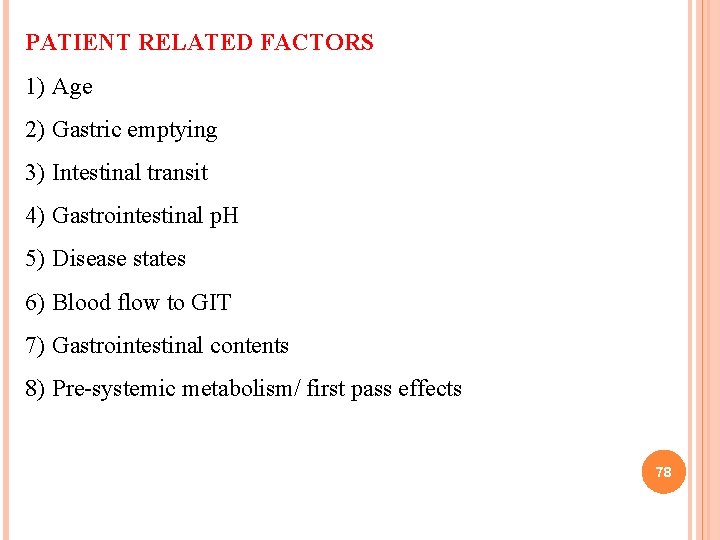
PATIENT RELATED FACTORS 1) Age 2) Gastric emptying 3) Intestinal transit 4) Gastrointestinal p. H 5) Disease states 6) Blood flow to GIT 7) Gastrointestinal contents 8) Pre-systemic metabolism/ first pass effects 78

GIT v. Stomach shows limited drug absorption ØThe total mucosal area is small ØThe epithelium is dominated by mucus- secreting rather than absorptive cells. ØThe gastric residence time is limited v. Lipophilic, neutral & acidic drugs are absorbed v. Small intestine major site for absorption v. Large surface area: The folds in intestinal mucosa (kerckring)↑ 3 folds. This folds posses finger like projections called as villi(↑ 30 times). From the surface of villi protrude microvilli ( 600 times) increase in surface area. 79
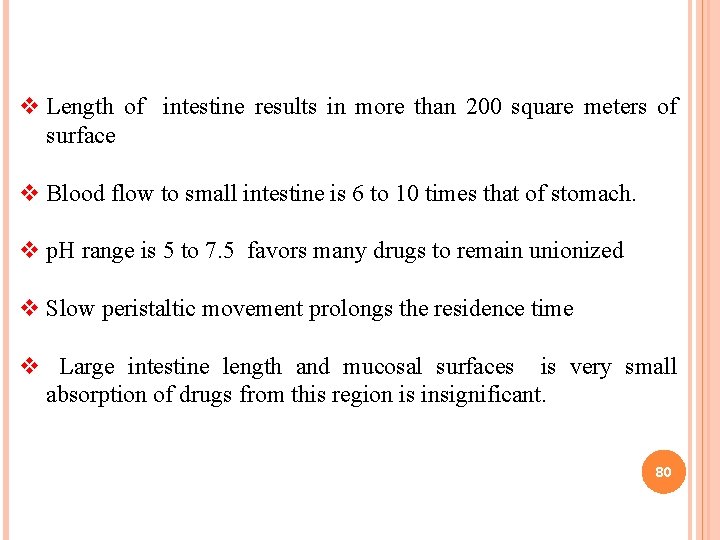
v Length of intestine results in more than 200 square meters of surface v Blood flow to small intestine is 6 to 10 times that of stomach. v p. H range is 5 to 7. 5 favors many drugs to remain unionized v Slow peristaltic movement prolongs the residence time v Large intestine length and mucosal surfaces is very small absorption of drugs from this region is insignificant. 80

v Absorption of water and electrolytes. v Long residence time (6 -12 hours) may be important in the absorption of some poorly soluble drugs and sustained release dosage forms 1. AGE v In infants the gastric p. H is high, intestine surface and blood flow is low v In the elderly altered gastric emptying , decreased intestinal surface area and GI blood flow & bacterial over growth shows altered absorption 81

2. GASTRIC EMPTYING v The passage of material from stomach to small intestine called as gastric emptying & is a first order process v Gastric emptying rate: is the speed at which stomach contents empty into the intestine v Gastric emptying time: is the time required for gastric contents to empty into the small intestine. Longer the gastric emptying rate slower the gastric emptying time v Gastric emptying : T 1/2 is the time taken to empty the stomach content by half. 82

Rapid gastric emptying is desirable v A rapid onset of action is desired e. g. sedatives v Dissolution of drug occurs in the intestine e. g. enteric coated v Drugs are unstable in gastric fluids e. g: penicillin G, erythromycin v Drug is best absorbed from distal part of the small intestine e. g. Vitamin B 12 83

Delay in gastric emptying desirable v Food promotes drug dissolution and absorption. Eg: griseofulvin v Disintegration and dissolution of dosage forms is promoted in gastric fluids v The drug dissolve slowly. e. g: griseofulvin v Drugs are absorbed from the proximal part of small intestine & prolonged drug-absorption site contact is desired. e. g. Vit B 2 & C 84
Factors Influence Gastric Emptying v Volume of meal : larger the bulk of the meal greater is the emptying time v Temperature of the meal : High or low temperature fluids in comparison with the body temperature reduces the gastric emptying rate v Composition of meal ü Carbohydrates>proteins>fats ü Fats promote secretion of bile which inhibits gastric emptying ü Fatty meal is beneficial for absorption of poorly soluble drugs like Gresiofulvin v Physical state and viscosity of meal: Liquids take about an hour 85 time & solid meal requires about 4 -5 hours for emptying.
v GI p. H : Gastric emptying is retarded at low gastric p. H & promoted at alkaline p. H. v Body posture: Gastric emptying is favored while standing & lying on the right side. v Emotional state: Stress & anxiety promotes gastric motility. Depression retards gastric motility. v Drugs: antacids, anticholinergics, narcotic analgesics retard while metoclopromide, domperidone & cisapride etc. stimulate GE 86
3. INTESTINAL TRANSIT v The residence time depends on the intestinal motility v Peristaltic movements promote absorption by increasing the drugintestinal membrane contact & enhancing the dissolution of poorly soluble drugs through induced agitation. v Delayed intestinal transit desirable Ø Drug that dissolves or releases slowly(sustained release) Ø Drugs that dissolve only in intestine (enteric coated formulations) Ø Drugs that are absorbed at specific sites in intestine(b vitamins) Ø Drug penetrates the mucosa very slowly ex: acyclovir v Food, decreased GI secretions, anticholinergic & pregnancy retard intestinal transit. v Diarrhoea, metoclopramide, laxatives promotes intestinal transit. 87

4) GASTROINTESTINAL p. H v Disintegration: enteric coated tablets made of p. H sensitive polymer like eudragits DT & Disso get effected. v Dissolution ü Most drugs are moderately acidic or basic ü p. H that favors formation of salt is desirable ü Weakly acidic drugs dissolve in alkaline p. H & weakly basic drugs dissolve in acidic p. H. v Absorption : depending on the drug pka & whether it is an acidic or basic drug, the GI p. H influences its ionization & drug absorption v Stability: acidic stomach p. H degrades penicillin G & erythromycin, counter by preparing pro-drugs carindacillin 88 & erythromycin estolate.
DISEASE STATES Gastrointestinal v Achlorhydria(decreased GI secretin and high p. H): decreases absorption of acidic drugs like Aspirin v Celiac disease: (destruction of villi & microvilli) v. Increased GI emptying rate v. Altered intestinal drug metabolism v. Steatorrhea: impaired secretion of bile , affecting absorption of lipophilic drugs. v Crohn’s disease: altered gut wall microbial flora, decreased gut 89 surface area & alter intestinal transit rate
v Cardiovascular : there is oedema of the intestine , decreased blood flow to GIT. It alters gastric emptying rate, gastric p. H, secretions, microbial flora. v Hepatic: cirrhosis influences the absorption of drugs that considerably undergo first pass metabolism. 90
6) BLOOD FLOW TO GIT v The high perfusion rate ensures drug permeated across membrane is rapidly removed from the absorption site maintaining sink conditions & concentration gradient. v. Lipid soluble drugs, it is the perfusion rate which is the rate limiting step in absorption. v. Food influences blood flow to GIT. The perfusion rate increases after meal & persists for few hours but drug absorption is not significantly influenced. 91

7) GASTROINTESTINAL CONTENTS i. Food-Drug interaction v Food may either delay, reduce, increase or may not affect absorption of drugs v General rule better absorbed in fasting condition & retarded in presence of food v Delayed /decreased absorption ü Delayed gastric emptying (enteric coated) & affecting the drugs unstable in stomach ex: penicillin ü High viscosity of meal prevents dissolution & diffusion of drug towards absorption site ü Forming of poorly soluble , un-absorbable complexes ex: 92 tetracycline-calcium

v Increased drug absorption ü Increased time for dissolution of poorly soluble drugs ü Enhanced solubility due to GI secretions like bile ü Prolonged residence time & absorption site contact of drug ex: water soluble vitamins ü Increased lymphatic absorption ii. Fluid Volume: v Large fluid volume results in better dissolution, rapid gastric emptying & enhanced absorption v Erythromycin is better absorbed when taken with glass of water under fasting conditions than when taken with meal 93

iii. Interaction of drug with normal GI constituents: v Mucin protective muco polysaccharide interacts streptomycin & certain quaternary ammonium compounds retard their absorption with v Bile salts ü Aid solubilisation & absorption of lipid soluble drugs ex: Gresiofulvin & Vit-A D E K ü Decreases absorption of Neomycin & Kanamycin by forming water insoluble complexes 94
iv. Drug-drug interactions : a) Physicochemical: v Adsorption: Anti diarrhoeals containing adsorbents (kaolin-pectin) retard / prevent absorption of drugs co-administered with them ex: Lincomycin v Complexation: Antacids containing heavy metals like Al, Ca, Fe, Mg or Zn form un-absorbable complex with Tetracyclin. v p. H Change: Basic drugs dissolve in acidic p. H. Co-administration of such drugs Tetracycline with antacids results in elevation of p. H hence low dissolution 95
b)Physiological: v Decreased GI Transit: Anticholinergics retard GI motility & promote absorption of drugs like ranitidine whereas delay of paracetamol absorption. v Increased Gastric Emptying: Metoclopromide promotes GI motility & enhance absorption of tetracycline & levodopa. v Altered GI Metabolism: antibiotics inhibit bacterial meatbolism of drugs e. g. erthromycin enhances efficacy of digoxin 96

7) FIRST PASS EFFECT/PRESYSTEMIC METABOLISM v Reasons for decreased oral bioavailability: 1) Decreased absorption (precipitation , complexation & poor solubility) 2) Destruction of the drug 3) First pass/ pre-systemic metabolism v. The loss of drug through biotransformation reaching before reaching systemic circulation after oral administration is first pass effect. 97

v. The three enzyme systems which affect pre-systemic metabolism of drug are ü Luminal enzymes • Digestive enzymes • Bacterial enzymes ü Gut wall or mucosal enzymes ü Hepatic enzymes Digestive enzymes: v Enzymes present in gut fluids including intestinal and pancreatic secretions. v Pancreatic contain hydrolases which hydolyse ester drugs like chloramphenicol palmitate to active chloramphenicol v Peptidases which split amide linkages hence inactivate protein drugs. 98 Peptides are delivered into colon (no peptidases)
Bacterial enzymes: v Present in stomach, small intestine & rich in colon. Render a drug more active or toxic on biotransformation. v E. g. Sulphasalazine (ulcerative colitis) hydrolyzed by these enzymes to sulphapyridine & 5 -amino salicylic acid. Gut wall/Mucosal enzymes: v Present in stomach, intestine & colon. ADH is an enzyme of stomach which inactivates alcohol v Intestinal mucosa contains both phase I & II & act on drugs e. g. sulfation of isoprenaline Hepatic enzymes: v Present in the liver responsible for first pass effect e. g. isoprenaline, nitroglycerine, morphine, lidocaine etc. 99
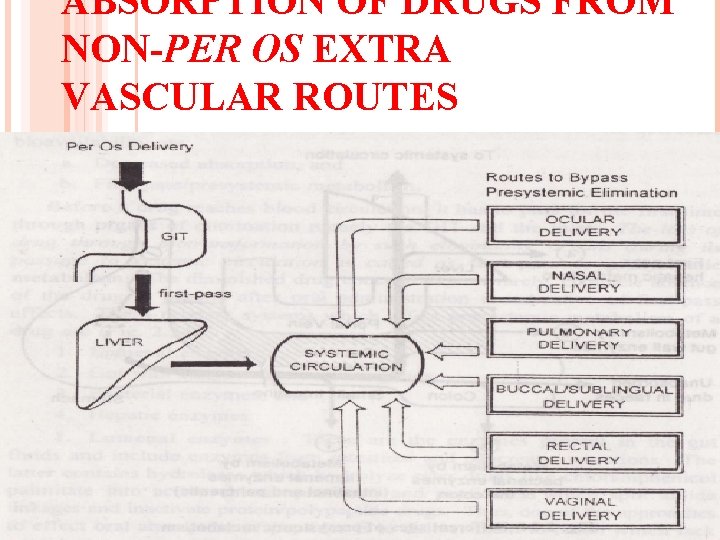
ABSORPTION OF DRUGS FROM NON-PER OS EXTRA VASCULAR ROUTES 100
Intraocular Administration v Meant for local effects such as mydriasis, miosis, anesthesia , glaucoma, etc v The barrier to penetration of drugs is the cornea which possesses both hydrophilic and lipophilic characteristics. v Optimum permeation occurs if drugs possess biphasic solubility. v p. H of the formulation influences lachrymal output—higher p. H decreases tear flow and promotes drug absorption & vice versa due to drug drainage 101

v Instillation of small volume of drug solution in concentrated form increases its effectiveness than when administered in large volume in dilute form v Viscosity imparters in the formulation increase bioavailability by prolonging drug’s contact time with the eye. 102
Intranasal Administration v Systemic delivery of peptide and protein drugs v Absorption is rapid as observed after parenteral administration because of its rich vasculature and high permeability v Lipophilic drugs, absorption by diffusion is observed upto 400 Daltons & satisfactory absorption up to 1000 Daltons. v Permeability enhancers (surfactants) added help absorption of drug with molecular weight of 6000 Daltons v Polar compounds primarily absorbed by pore transport(200 Daltons) v 103 Nasal permeation is effected by p. H of nasal secretions (5. 5 to 6. 5), viscosity, pathological conditions such as common cold and rhinitis
Pulmonary Administration v Large surface area of the alveoli, high permeability of the alveolar epithelium and rich perfusion permit extremely rapid absorption v Route limited for administering drugs affecting pulmonary system bronchodilators , anti-inflammatory steroid & antiallergics v Lipophilic drugs are rapidly absorbed by passive diffusion and polar drugs by pore transport v p. H of pulmonary fluids, particle size of the aerosolised droplets (0. 6 microns) from which drug absorption is rapid, penetrate rapidly but are susceptible to easy exhalation v 104 Patients’ inability to inhale a sufficient amount of drug is the limitation
SUBLINGUAL / BUCCAL ROUTE v Sublingual route: Dosage form is placed beneath the tongue. v Buccal route: Dosage form is placed between the cheek and teeth. v Drugs administered by this route are supposed to produce systemic action act because as the absorbed drug drains directly into the general circulation. v Oral mucosal regions are highly vascularized therefore rapid onset of action is observed. Eg, Nitroglycerin Oxytocin, Fenosterol etc. 105

Factors to be considered: v Lipid solubility should be high for absorption with a low dose v Drug should be soluble in buccal fluid & p. H of saliva which is 6 v Sublingual absorption is faster than buccal, because mucosa region is thinner than that of buccal mucosa v Limited mucosal surface area. v Taste of medicament and discomfort. 106
RECTAL ADMINISTRATION v This route of administration is useful in children, old people and unconscious patients & occurs by passive diffusion. v Drugs may be administered as solutions (microenemas) or suppositories v Irritating suppository bases such as PEG promotes defecation and drug loss. v Highly vascularized, absorption is slower because of limited surface area. v Eg. , drugs that are administered are: aspirin, acetaminophen, theophylline, indomethacin, promethazine & certain barbiturates. 107

VAGINAL ADMINISTRATION v Generally intended to act locally in treatment of bacterial or fungal infections or prevent conception v Systemic delivery of contraceptives & steroids with no first-pass metabolism v Buffering, size & shape of dosage a must for better patient convenience & compliance v p. H of lumen fluids (4 to 5), vaginal secretions and the microorganisms present in the vaginal lumen which may metabolize the drug may influence absorption 108
Topical Administration v Drugs applied topically are meant to exert their effect locally & drugs that exert their effects systemically, the mode of administration is called as percutaneous or transdermal delivery. v Anatomically, the skin is made of 3 distinct layers ü Epidermis is the nonvascular, multilayered outer region of the skin. ü Dermis or true skin is a highly vascular region; drugs permeating to this region are taken up into the systemic circulation ü subcutaneous fat tissue v Principal barrier to the entry of drugs is the most superficial layer 109 of epidermis called as stratum corneum

Factors influencing absorption v Absorption is very slow from regions such as foot and palm where the skin has thickened stratum corneum. v Absorption is rapid from regions where numerous hair follicles exist e. g. scalp. v Rashes, inflammation, mild burns stratum corneum is destroyed, promote drug absorption. v Hydration of skin promote hydration of skin and drug absorption. 110
Novel techniques: Ionic drugs are not absorbed transdermally, absorption of such drugs can be affected by the following Iontophoresis: v Drug delivery into the body by means of an electric current v An ionized drug in solution is placed on the skin and an electrical potential difference established thus driving the ions into the skin. v Application of a positive current will drive positively charged drug molecules away from the electrode and into the tissues & vice versa e. g. Cortisol, methacholine, Phonophoresis: Defined as the movement of drug molecules through 111 the skin under the influence of ultrasound.

May 2009 1. Write the factors influencing drug absorption through GIT with examples(15) 2. What is Noyes-Whitney’s equation? (2) May 2010 1. Explain the Noyes-whitney’s equation(5) 2. Explain the mechanism of drug absorption by passive diffusion(5) 3. Define dissolution and name theories of drug dissolution (2). 4. List the different mechanisms of drug absorption(2) 5. State the Fick’s law of diffusion and give its mathematical expression(2) 112

May 2011 1. Describe p. H partition hypothesis (5) 2. Explain the Noise-whitney’s equation (5) 3. Explain Fick’s first law of diffusion(2) 4. How does food affect the rate and extent of drug absorption from GIT? (2) 5. How do bacteria present in the colon influence colonic p. H (2) 6. What is endocytosis? (2) May 2012 1. Define absorption. Explain the various biological, physicochemical and pharmaceutical factors which affect the drug absorption(15) 2. What is Hasselbalch equation(2) 3. What do you mean by rate-limited step in drug absorption? (2) 113

May 2013 1. Explain the various physic-chemical and biological factors affecting gastro-intestinal absorption of drugs(15) 2. Fick’s first law of diffusion(2) 114
- Slides: 114