Fabry Disease new therapies in 2019 Dr Michael

Fabry Disease: new therapies in 2019 Dr. Michael L. West Division of Nephrology Department of Medicine Dalhousie University Halifax NS Canada mlwest@dal. ca
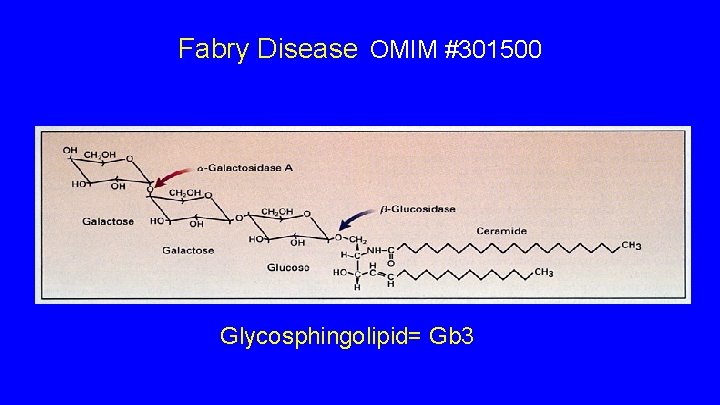
Fabry Disease OMIM #301500 Glycosphingolipid= Gb 3
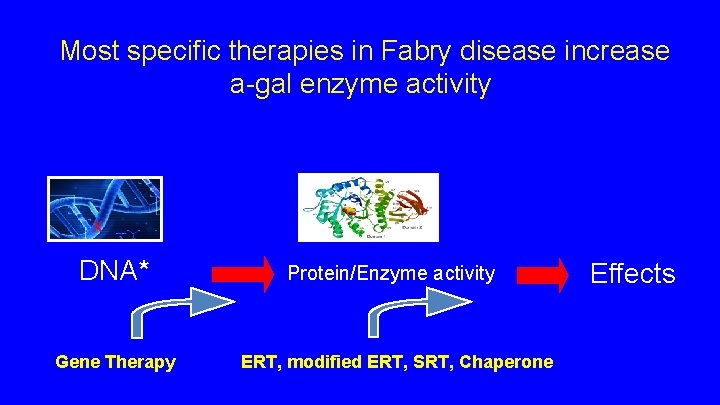
Most specific therapies in Fabry disease increase a-gal enzyme activity X DNA* Gene Therapy Protein/Enzyme activity ERT, modified ERT, SRT, Chaperone Effects

New Therapies Chaperone Modified ERT Substrate Reduction Therapy Gene Therapy

Migalastat Chaperone (Galafold™, Amicus) • • oral every 2 days licensed in Canada 2017 well tolerated no problems with infusion reactions, antibodies • decreased heart wall thickness in some after switched from ERT to Migalastat • Recent reports of clearing of cornea verticillata
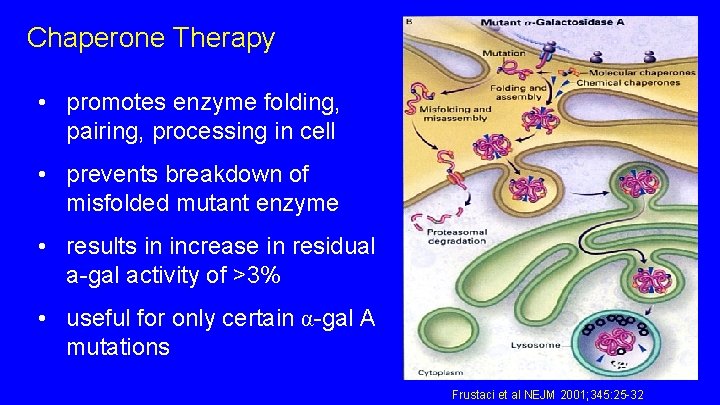
Chaperone Therapy • promotes enzyme folding, pairing, processing in cell • prevents breakdown of misfolded mutant enzyme • results in increase in residual a-gal activity of >3% • useful for only certain α-gal A mutations Frustaci et al NEJM 2001; 345: 25 -32

Issues with Migalastat Chaperone • • • reimbursed in almost all provinces in Canada private insurance coverage costly must have mutation that is “amenable” to chaperone only 25% Fabry patients in Canada eligible must also meet Canadian Fabry treatment guidelines, same as for ERT • adults only
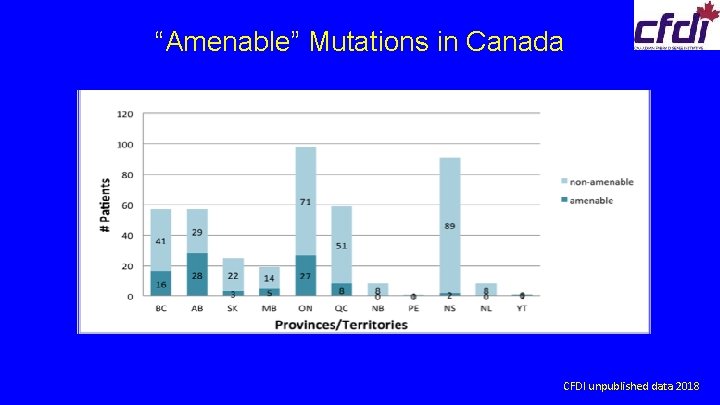
“Amenable” Mutations in Canada CFDI unpublished data 2018
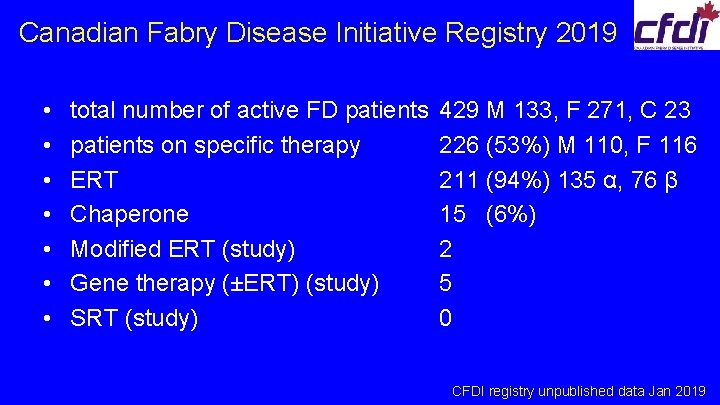
Canadian Fabry Disease Initiative Registry 2019 • • total number of active FD patients on specific therapy ERT Chaperone Modified ERT (study) Gene therapy (±ERT) (study) SRT (study) 429 M 133, F 271, C 23 226 (53%) M 110, F 116 211 (94%) 135 α, 76 β 15 (6%) 2 5 0 CFDI registry unpublished data Jan 2019
Ongoing Migalastat Studies (Amicus) • Migalastat registry to determine long term outcomes • Switch studies from ERT (Replagal, Fabrazyme) to Migalastat • Study of biomarkers in blood and urine to determine Migalastat effect • Study of Migalastat in children (no sites in Canada) Search clinicaltrials. gov Contact: Amicus Therapeutics Patient Advocacy 609 -662 -2000; clinicaltrials@amicusrx. com
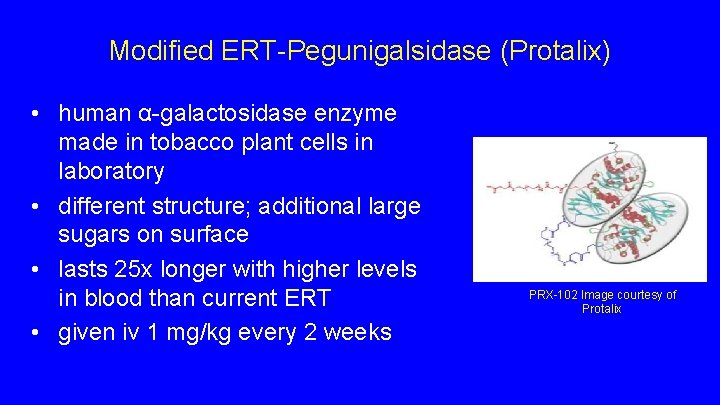
Modified ERT-Pegunigalsidase (Protalix) • human α-galactosidase enzyme made in tobacco plant cells in laboratory • different structure; additional large sugars on surface • lasts 25 x longer with higher levels in blood than current ERT • given iv 1 mg/kg every 2 weeks PRX-102 Image courtesy of Protalix

Modified ERT PRX-102 Image courtesy of • Pegunigalsidase alfa (PRX-102) Protalix • more stable, may cause less antidrug antibodies, safe, effective, well tolerated • phase III studies in adults-Canada, US, EU, Aus • not licensed anywhere
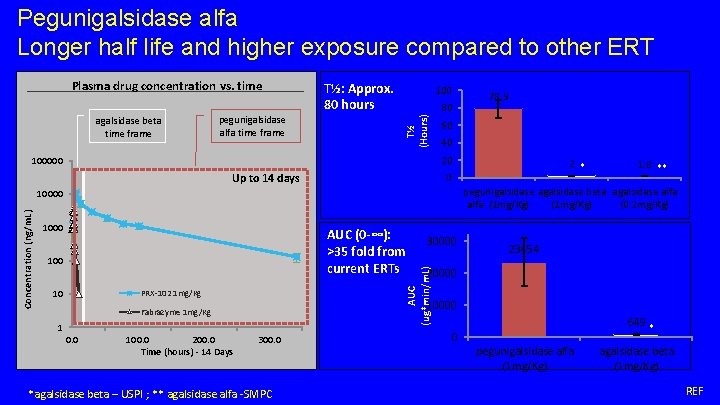
Pegunigalsidase alfa Longer half life and higher exposure compared to other ERT agalsidase beta time frame pegunigalsidase alfa time frame T½: Approx. 80 hours 100 80 T½ (Hours) Plasma drug concentration vs. time 60 40 20 100000 Up to 14 days 1000 1 100. 0 200. 0 Time (hours) - 14 Days 23454 20000 10000 Fabrazyme 1 mg/Kg 0. 0 30000 AUC (ug*min/m. L) PRX-102 1 mg/Kg 10 1. 8 ** pegunigalsidase agalsidase beta agalsidase alfa (1 mg/Kg) (0. 2 mg/Kg) AUC (0 -∞): >35 fold from current ERTs 100 2 * 0 10000 Concentration (ng/m. L) 78. 9 300. 0 *agalsidase beta – USPI ; ** agalsidase alfa -SMPC 649 * 0 pegunigalsidase alfa (1 mg/Kg) agalsidase beta (1 mg/Kg) REF

Pegunigalsidase Results • • BRIDGE study adults, prior ET agalsidase alfa x 2 yrs 16 pts treated w pegunigalsidase 1 mg/kg iv every 2 wks e. GFR improved from -6. 8 to +3. 7 ml/min/1. 73 m 2 Preliminary data, of 16/22 subjects, unpublished
Ongoing Modified ERT Studies • BRIDGE-adults switched from Replagal to Pegunigalsidase every 2 wks iv • BALANCE-adults switched from Fabrazyme to Pegunigalsidase every 2 wks iv • BRIGHT-adults taking Pegunigalsidase double dose every 4 wks iv • BRILLIANCE-long term extension study 2 years • Halifax is only site in Canada

Substrate Reduction Therapy SRT • inhibitor of enzyme upstream from a-galactosidase • Shown to decreases build up of Gb 3 • Lucerastat (Idorsia) • oral, well tolerated • Phase III study Fabry patients with neuropathic pain, switch from ERT to SRT • Canadian study sites Halifax, Winnipeg, Vancouver • Web site for prospective subjects https: //www. modifyfabry. com
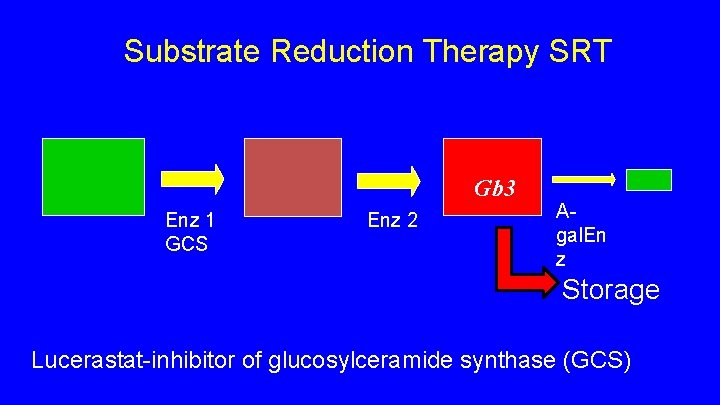
Substrate Reduction Therapy SRT Gb 3 Enz 1 GCS Enz 2 Agal. En z Storage Lucerastat-inhibitor of glucosylceramide synthase (GCS)
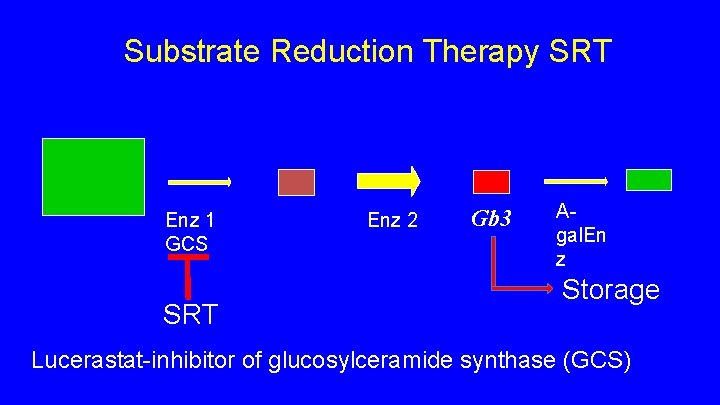
Substrate Reduction Therapy SRT Enz 1 GCS SRT Enz 2 Gb 3 Agal. En z Storage Lucerastat-inhibitor of glucosylceramide synthase (GCS)
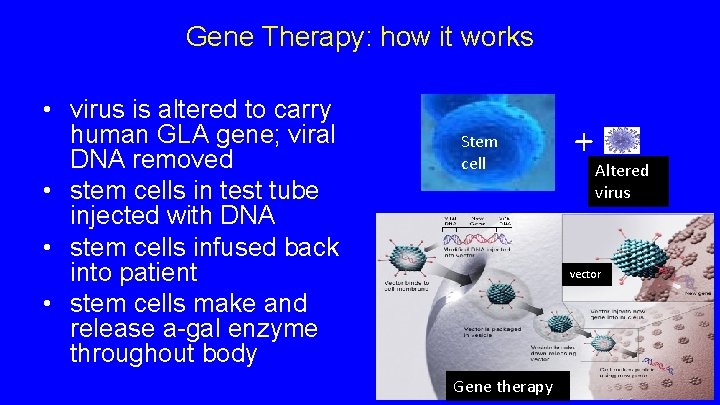
Gene Therapy: how it works • virus is altered to carry human GLA gene; viral DNA removed • stem cells in test tube injected with DNA • stem cells infused back into patient • stem cells make and release a-gal enzyme throughout body Stem cell + Altered virus vector Gene therapy

Why Gene Therapy for Fabry Disease? • single gene defect • small DNA • mice created with gene therapy are healthy with >10, 000 -fold higher a-gal A activity
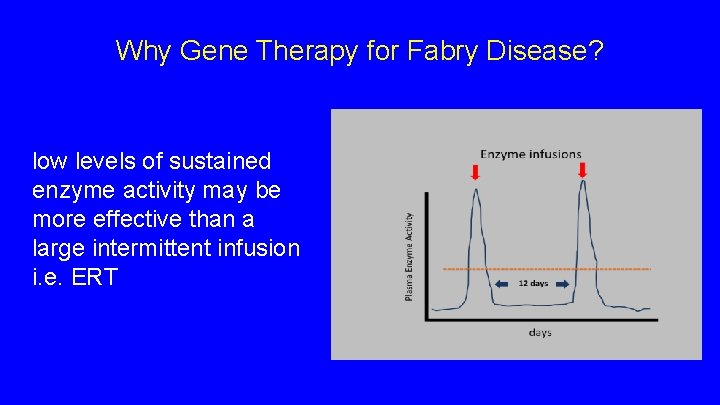
Why Gene Therapy for Fabry Disease? low levels of sustained enzyme activity may be more effective than a large intermittent infusion i. e. ERT
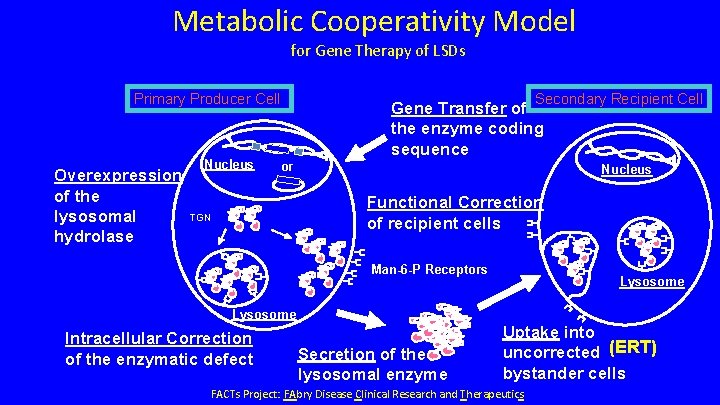
Metabolic Cooperativity Model for Gene Therapy of LSDs Primary Producer Cell Overexpression of the lysosomal hydrolase Nucleus Secondary Recipient Cell Gene Transfer of the enzyme coding sequence or Nucleus Functional Correction of recipient cells TGN Man-6 -P Receptors Lysosome Intracellular Correction of the enzymatic defect Secretion of the lysosomal enzyme Uptake into uncorrected (ERT) bystander cells FACTs Project: FAbry Disease Clinical Research and Therapeutics
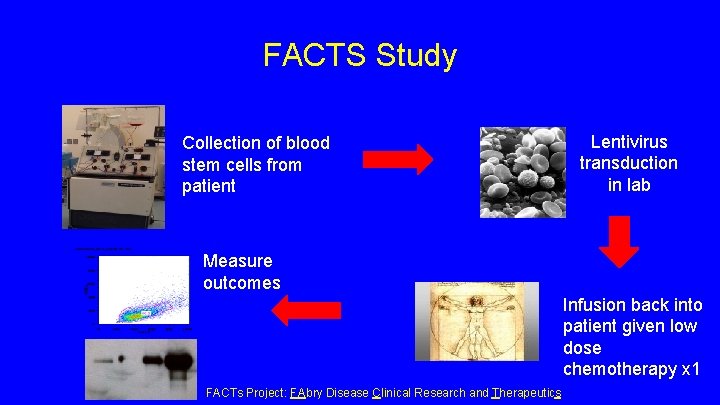
FACTS Study Collection of blood stem cells from patient Lentivirus transduction in lab Measure outcomes Infusion back into patient given low dose chemotherapy x 1 FACTs Project: FAbry Disease Clinical Research and Therapeutics
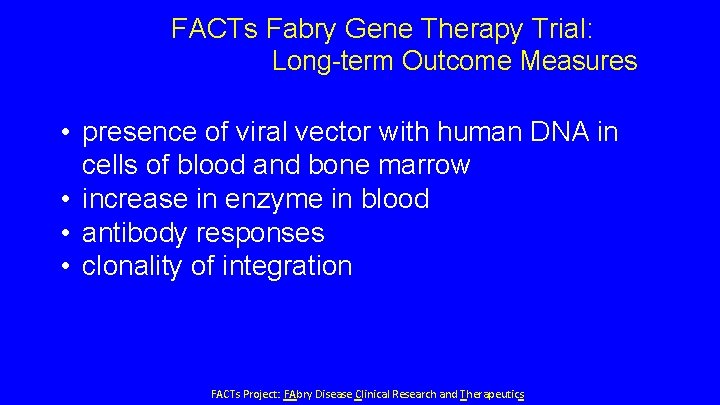
FACTs Fabry Gene Therapy Trial: Long-term Outcome Measures • presence of viral vector with human DNA in cells of blood and bone marrow • increase in enzyme in blood • antibody responses • clonality of integration FACTs Project: FAbry Disease Clinical Research and Therapeutics

Additional Drug Regimen • Drug conditioning to make space in BM (melphalan 100 mg/m 2 iv x 1) • with metabolic cooperativity effects, do not need 100% donor cells transfected • out-patient procedure: well tolerated by Fabry patients FACTs Project: FAbry Disease Clinical Research and Therapeutics

FACTS study • Health Canada approval April 29, 2016 • 5 patients enrolled, last in Calgary Feb 2019 • Enrollment finished Nov. 2018: patient 4 during infusion of transduced cells in Halifax Permission was obtained to use patient image

FACTS Study • 5 adult males underwent SCT after LV/AGA treatment • monitor patients closely for adverse effects to determine safety • followed by Fabry specialist and bone marrow specialist for 5 years FACTs Project: FAbry Disease Clinical Research and Therapeutics

Permission has been obtained to use patient image al Research and Therapeutics

Outcomes • Patients all feeling well • All with increased a-gal activity in plasma and white blood cells • All with new GLA gene present in their DNA in white blood cells FACTs Project: FAbry Disease Clinical Research and Therapeutics

Safety Adverse Events • none related to viral vector • transient low blood counts week 2 in all patients 3 patients: adverse effects related to protocol but not viral vector • fainting spell • bled into thigh with low platelet count • fever with low WBC, FACTs Project: FAbry Disease Clinical Research and Therapeutics

FACTs trial offers several firsts • first in the world Fabry disease gene therapy trial • first adult LV gene therapy trial in lysosomal diseases 1

Questions about Gene Therapy • How long can transduced stem cells live and function in the body? • Can stem cells make enough enzyme to keep patients healthy? • Is this procedure safe in the long term? • Will gene therapy cost less than ERT?

Avro. Bio gene study • • • based on same vector 12 male Fabry patients ages 16 -55; 2 done already no prior ERT kidney biopsies Australia, USA, Calgary, Halifax in Canada
Other gene studies • AAV adeno-associated virus, iv infusion x 1; targeted to liver cells, make enzyme, increase a-gal activity in kidney, heart and liver in Fabry mouse studies (Sangamo, USA), recruiting patients now • AAV 8, similar study in mice w increased a-gal activity, decr GB 3 in organs (Freeline, UK) • AAV targeted to heart, study in mice, monkeys, (4 DMT, USA) • AAV 9 study in mice, increased a-gal activity in organs (Niu, Taiwan) • m. RNA iv, makes enzyme, study in mice (Moderna, USA)

New ERT Studies • Switch from chaperone Migalastat or Replagal ERT to Fabrazyme ERT (Sanofi-Genzyme) • Adult males on therapy <5 years • Kidney biopsy x 2 • ? any study sites in Canada

New ERT Studies • Replagal ERT increased dose (Takeda) • No details yet • ? any study sites in Canada
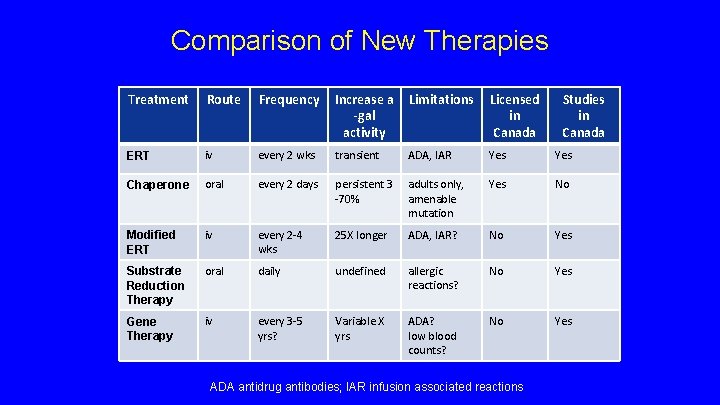
Comparison of New Therapies Treatment Route Frequency Increase a -gal activity Limitations Licensed in Canada ERT iv every 2 wks transient ADA, IAR Yes Chaperone oral every 2 days persistent 3 -70% adults only, amenable mutation Yes No Modified ERT iv every 2 -4 wks 25 X longer ADA, IAR? No Yes Substrate Reduction Therapy oral daily undefined allergic reactions? No Yes Gene Therapy iv every 3 -5 yrs? Variable X yrs ADA? low blood counts? No Yes ADA antidrug antibodies; IAR infusion associated reactions Studies in Canada
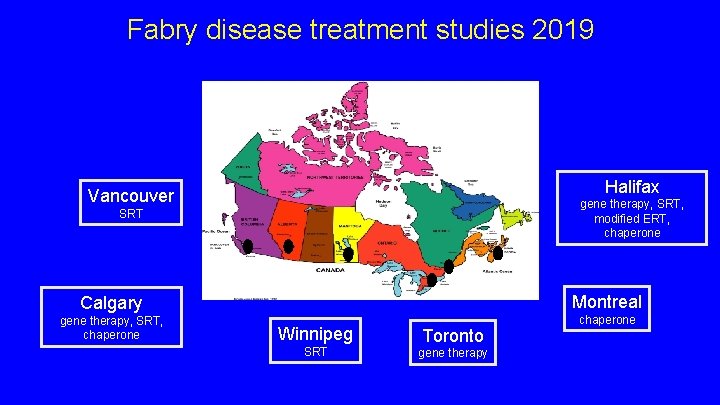
Fabry disease treatment studies 2019 Halifax Vancouver gene therapy, SRT, modified ERT, chaperone SRT Calgary Montreal gene therapy, SRT, chaperone Winnipeg SRT Toronto gene therapy

Conclusions • ERT and chaperone are only licensed therapies to date for Fabry disease in Canada • Still awaiting a cure • Many new therapies being developed: – – modified ERT substrate reduction therapy gene therapy other • Treatment and outcomes will only get better with studies of new agents
- Slides: 39