F Bonding and Chemical Formulas Ionic Compounds Why

F Bonding and Chemical Formulas Ionic Compounds

Why are some elements so reactive (e. g. Na) and others inert (e. g. noble gases)?

Valence Electrons Valence electrons are the electrons in the highest occupied energy level of the atom. Valence electrons are the only electrons generally involved in bond formation.

Valence electrons The number of valence electrons is related to the group number 1 A – 1 valence electron 4 A – 4 valence electrons 7 A – 7 valence electrons

Atoms tend to lose or gain e- in such a way as to achieve the electronic configurations of the nearest noble gases (The Octet Rule or Duet Rule)
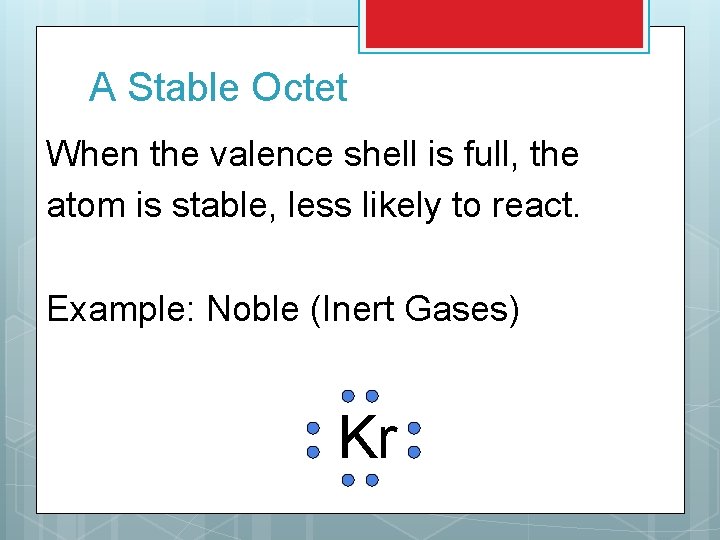
A Stable Octet When the valence shell is full, the atom is stable, less likely to react. Example: Noble (Inert Gases) Kr
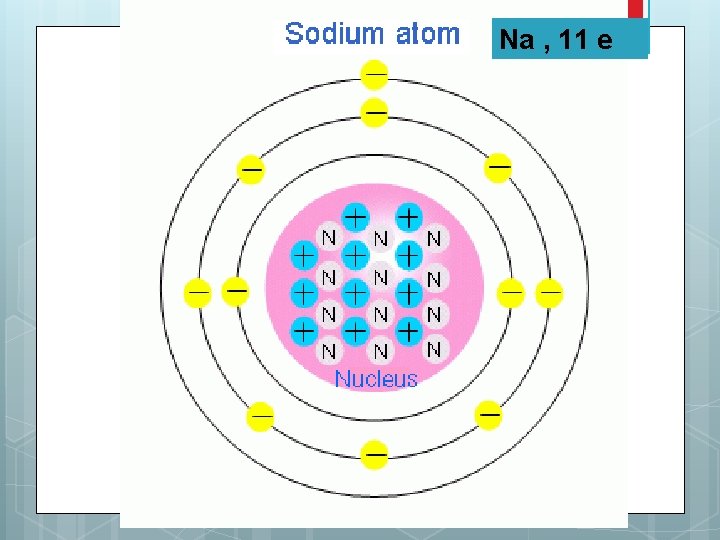
Na , 11 e

Electron Configuration of Sodium 1 s 2 2 p 6 3 s 1 Which is the valence electron for Na? Answer: 1 s 2 2 p 6 3 s 1

Sodium forming a cation Sodium has e- configuration of 1 s 2 2 p 6 3 s 1 Losing It its valence e- 1 s 2 2 p 6 now has the e- configuration of neon with a + 1 charge Na+
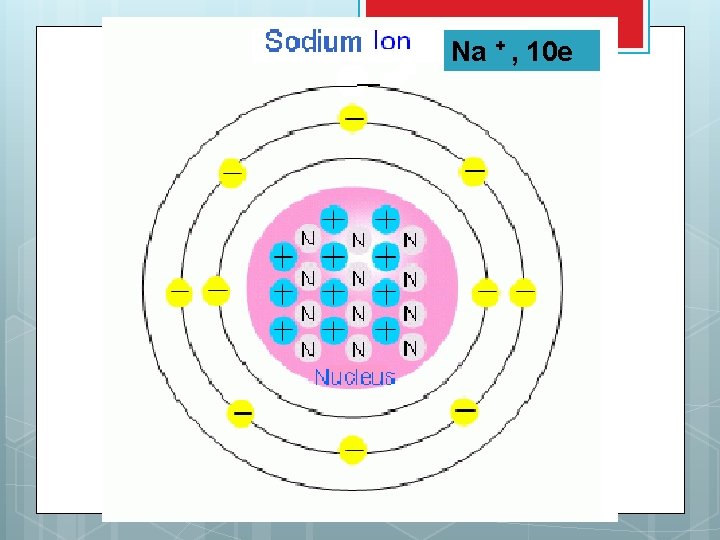
Na + , 10 e

Electron Configurations for Cations • • An atom’s loss of valence electrons produces a cation (positively charged ion) Most metals atoms have one to three valence electrons, which are easily removed (low ionization energy)

Electron Configurations for Anions • The gain of negatively charged electrons by a neutral atom produces anions Cl has e- configuration of 1 s 22 p 63 s 23 p 5 To become like argon, Cl would gain 1 e- and have the configuration 1 s 22 p 63 s 23 p 6 Chlorine would now have a negative charge Cl-
Lewis Structures for ionic compounds Write symbols and Lewis dot structures Draw an arrow to show the transfer of electrons Write each new ion along with it’s charge Write neutral ionic formula
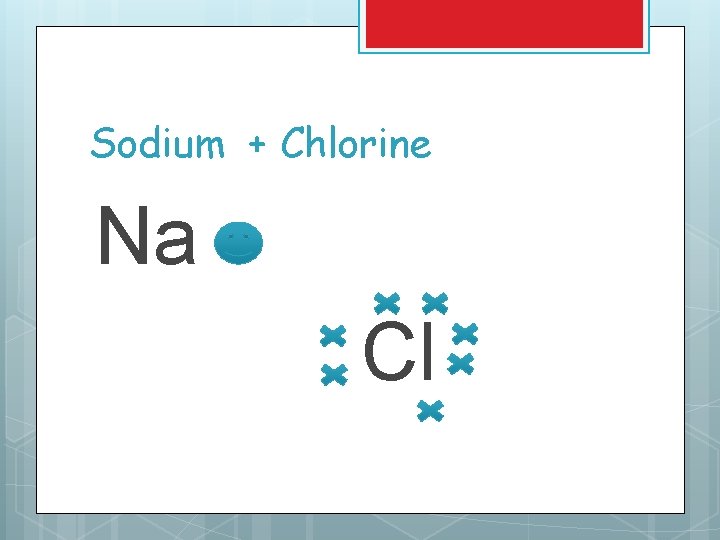
Sodium + Chlorine Na Cl
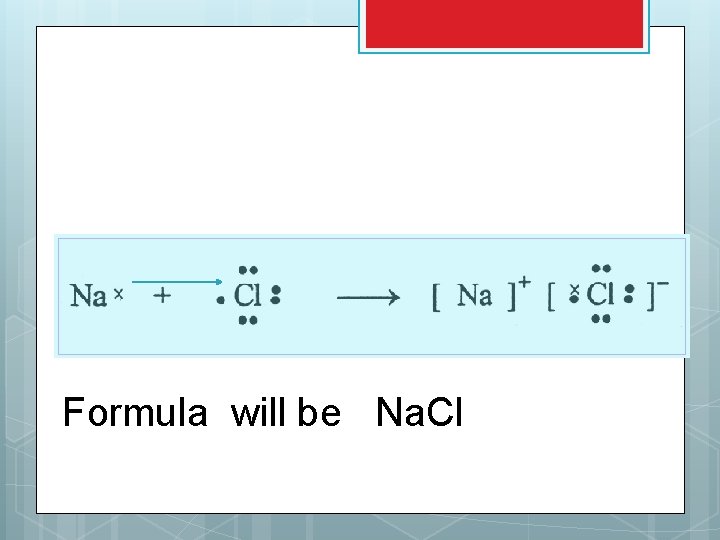
Formula will be Na. Cl
- Slides: 15