F 6 Chemistry Project Dipoledipole interaction and its


- Slides: 15

F. 6 Chemistry Project Dipole-dipole interaction and its application Fung Chun Yu (8) Kwok Chun Nga (9) Chan Wai Sum (5)

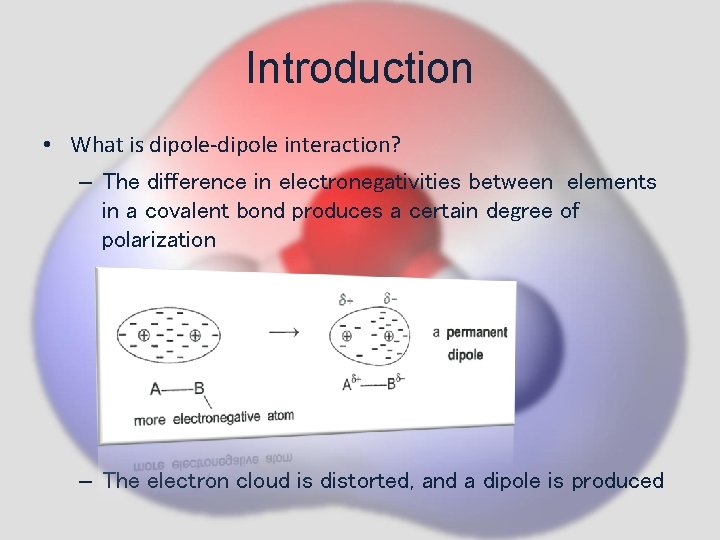
Introduction • What is dipole-dipole interaction? – The difference in electronegativities between elements in a covalent bond produces a certain degree of polarization – The electron cloud is distorted, and a dipole is produced

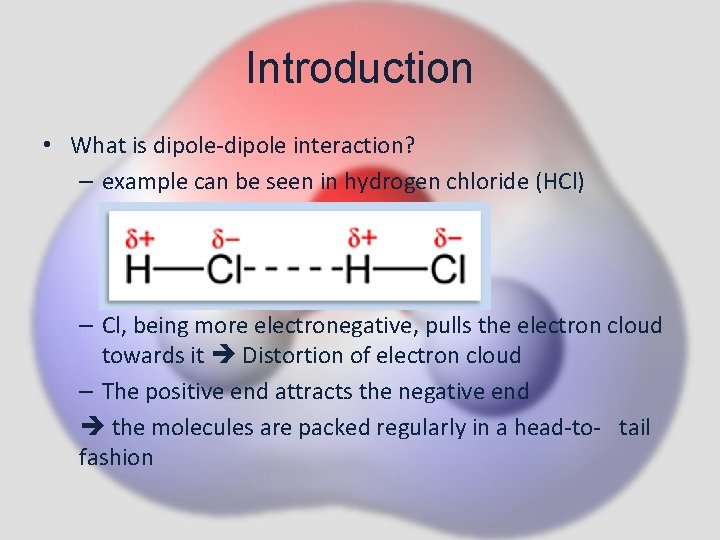
Introduction • What is dipole-dipole interaction? – example can be seen in hydrogen chloride (HCl) – Cl, being more electronegative, pulls the electron cloud towards it Distortion of electron cloud – The positive end attracts the negative end the molecules are packed regularly in a head-to- tail fashion

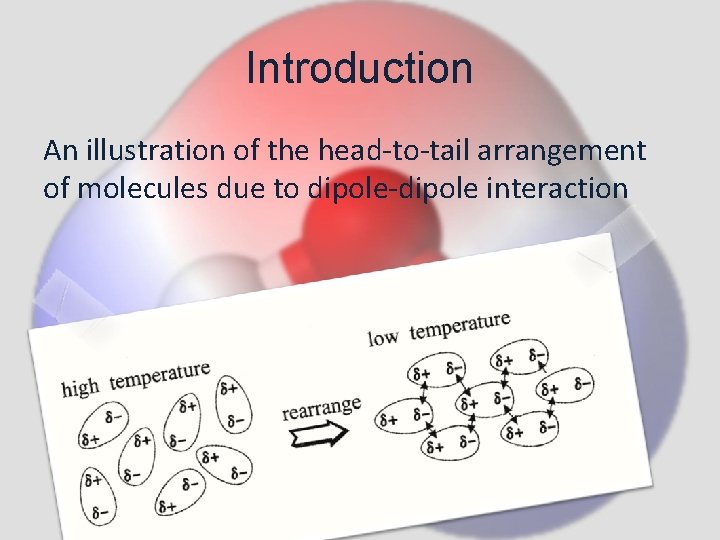
Introduction An illustration of the head-to-tail arrangement of molecules due to dipole-dipole interaction

Introduction An extraordinarily simple animation showing the interaction between HCl molecules

Application of dipole-dipole interaction – LCD – Biological field

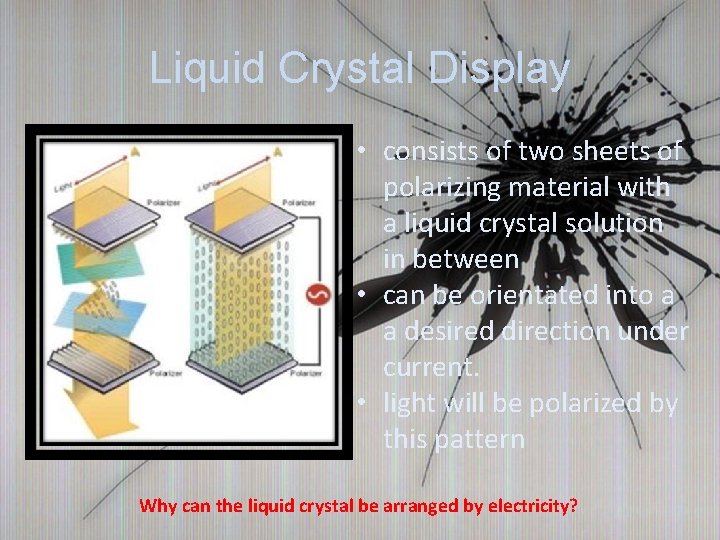
Liquid Crystal Display • consists of two sheets of polarizing material with a liquid crystal solution in between • can be orientated into a a desired direction under current. • light will be polarized by this pattern Why can the liquid crystal be arranged by electricity?

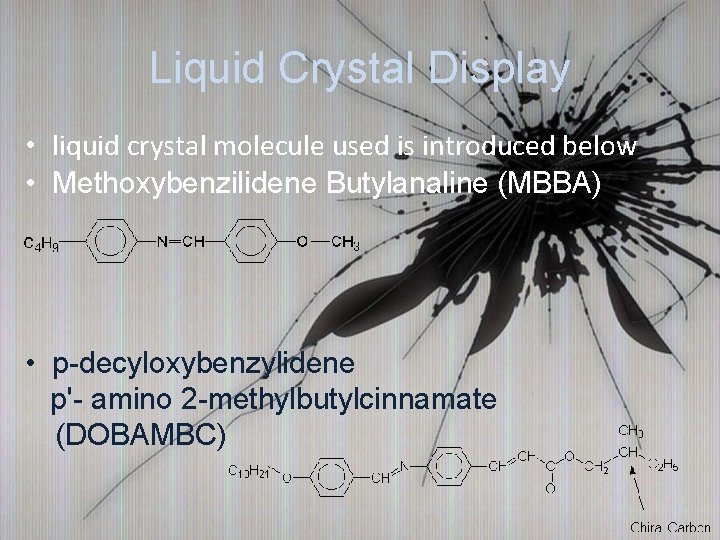
Liquid Crystal Display • liquid crystal molecule used is introduced below • Methoxybenzilidene Butylanaline (MBBA) • p-decyloxybenzylidene p'- amino 2 -methylbutylcinnamate (DOBAMBC)

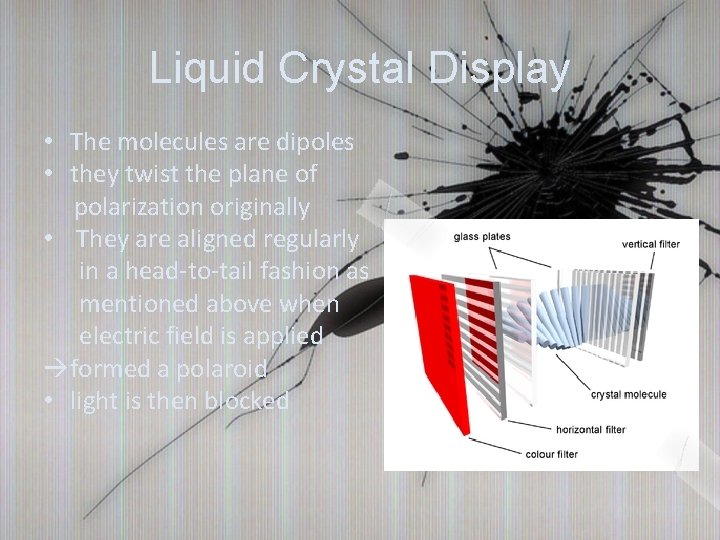
Liquid Crystal Display • The molecules are dipoles • they twist the plane of polarization originally • They are aligned regularly in a head-to-tail fashion as mentioned above when electric field is applied àformed a polaroid • light is then blocked

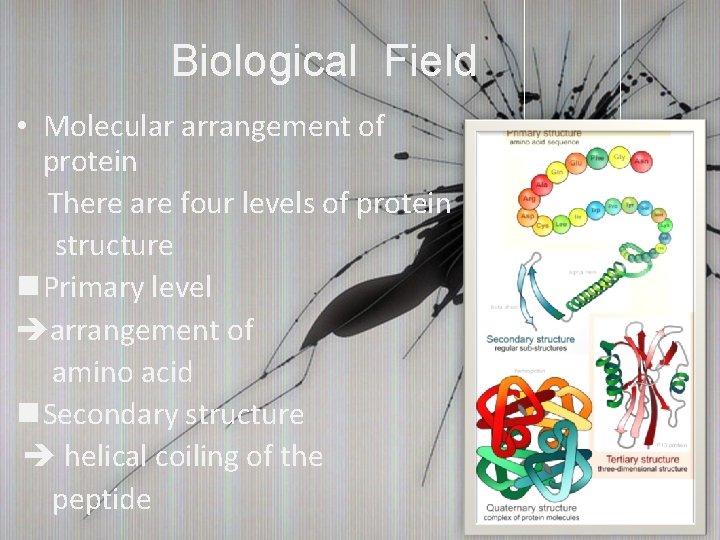
Biological Field • Molecular arrangement of protein There are four levels of protein structure n Primary level arrangement of amino acid n Secondary structure helical coiling of the peptide

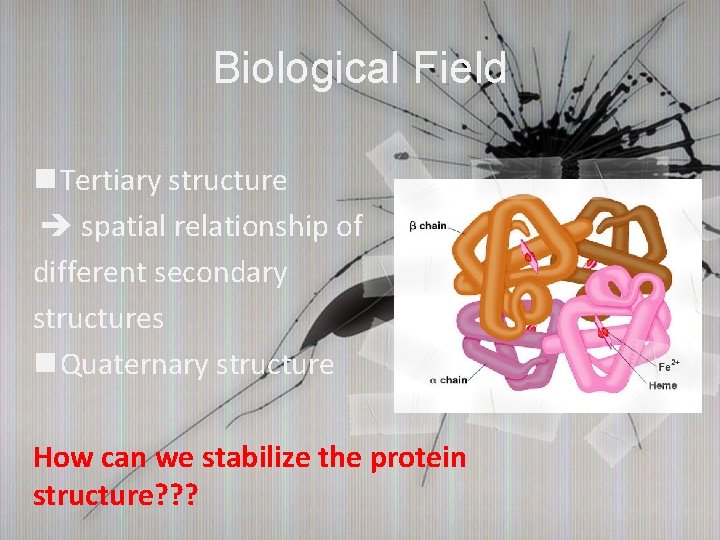
Biological Field n Tertiary structure spatial relationship of different secondary structures n Quaternary structure How can we stabilize the protein structure? ? ?

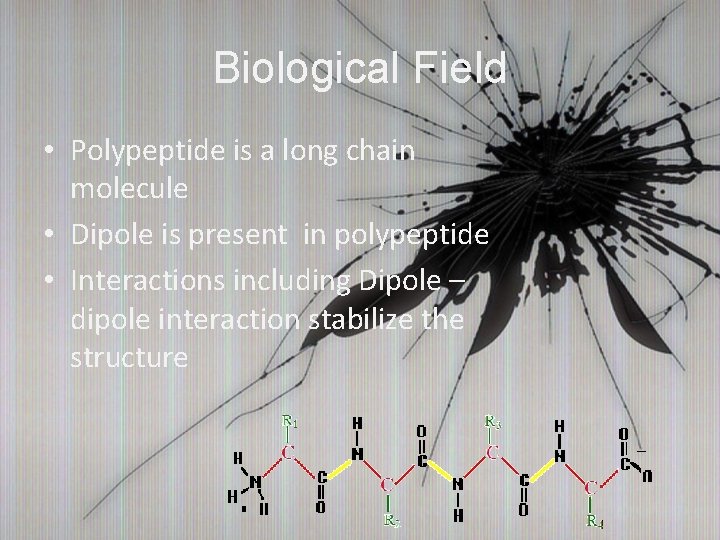
Biological Field • Polypeptide is a long chain molecule • Dipole is present in polypeptide • Interactions including Dipole – dipole interaction stabilize the structure

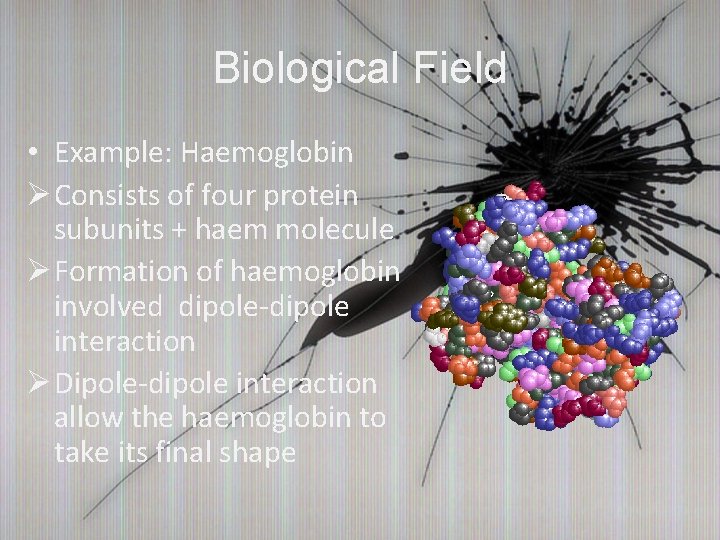
Biological Field • Example: Haemoglobin Ø Consists of four protein subunits + haem molecule Ø Formation of haemoglobin involved dipole-dipole interaction Ø Dipole-dipole interaction allow the haemoglobin to take its final shape

Acknowledgement • Wikipedia (http: //en. wikipedia. org/ • http: //www. gullwinguk. com • http: //bucarotechelp. com/computers/anatom y/91060003. asp

The End