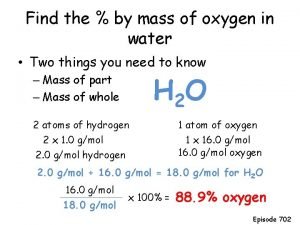F 5351 Metalloproteins reacting with oxygen 1 Why

![3 O 2 + 1[L 5 Fe] 3[L 5 Fe. O 2] spin-allowed: n° 3 O 2 + 1[L 5 Fe] 3[L 5 Fe. O 2] spin-allowed: n°](https://slidetodoc.com/presentation_image/1f1d9308c47edebf9c08aecd8e739791/image-13.jpg)
![3 O 2 + 1[L 5 Fe] 3[L 5 Fe. O 2] spin-allowed: n° 3 O 2 + 1[L 5 Fe] 3[L 5 Fe. O 2] spin-allowed: n°](https://slidetodoc.com/presentation_image/1f1d9308c47edebf9c08aecd8e739791/image-14.jpg)
![3 O 2 + 1[L 5 Fe] 3[L 5 Fe. O 2] spin-allowed: n° 3 O 2 + 1[L 5 Fe] 3[L 5 Fe. O 2] spin-allowed: n°](https://slidetodoc.com/presentation_image/1f1d9308c47edebf9c08aecd8e739791/image-15.jpg)


![Stretching frequencies and bond lengths in dioxygen species Species [A] n. O-O [cm-1] d Stretching frequencies and bond lengths in dioxygen species Species [A] n. O-O [cm-1] d](https://slidetodoc.com/presentation_image/1f1d9308c47edebf9c08aecd8e739791/image-29.jpg)





![Functional hemocyanin models [(tmpa)2 Cu 2 O 2]2+ [Cu{HB(3, 5 -i. Pr 2 pz)3}]2(O Functional hemocyanin models [(tmpa)2 Cu 2 O 2]2+ [Cu{HB(3, 5 -i. Pr 2 pz)3}]2(O](https://slidetodoc.com/presentation_image/1f1d9308c47edebf9c08aecd8e739791/image-53.jpg)

- Slides: 62

F 5351 Metalloproteins reacting with oxygen 1. Why do aerobic organisms need metalloproteins? 2. Oxygen transport proteins & Oxygenases 2. 1. Hemoglobin, Myoglobin & Cytochrome P 450 2. 2. Hemerythrin & Methane monooxygenase 2. 3. Hemocyanin & Tyrosinase 3. Conclusion Jiří Kozelka 13. 11. 2014 kozelka. jiri@gmail. com

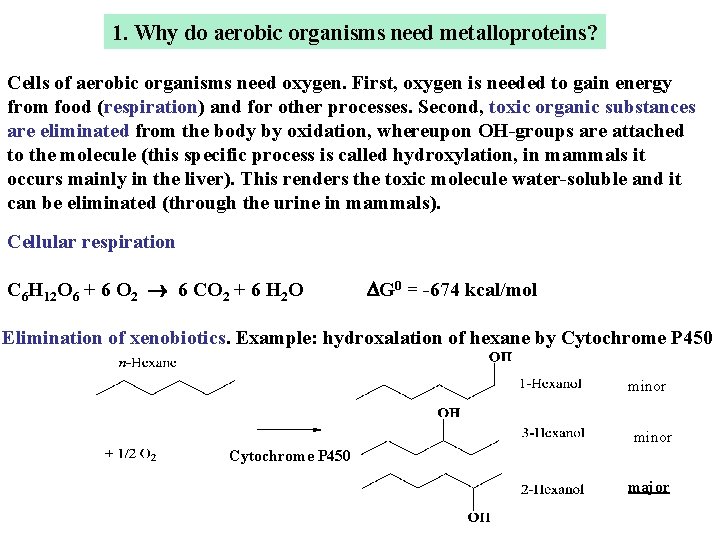
1. Why do aerobic organisms need metalloproteins? Cells of aerobic organisms need oxygen. First, oxygen is needed to gain energy from food (respiration) and for other processes. Second, toxic organic substances are eliminated from the body by oxidation, whereupon OH-groups are attached to the molecule (this specific process is called hydroxylation, in mammals it occurs mainly in the liver). This renders the toxic molecule water-soluble and it can be eliminated (through the urine in mammals). Cellular respiration C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O DG 0 = -674 kcal/mol Elimination of xenobiotics. Example: hydroxalation of hexane by Cytochrome P 450 minor Cytochrome P 450 major

Use of oxygen by aerobic organisms is hampered by two problems: 1. The solubility problem Water solubility of oxygen at 25 o. C and pressure = 1 bar is at 40 mg/L water. This is not enough to guarantee the oxygen supply to mitochondria by mere diffusion. Cells of aerobic organisms use therefore oxygen transporters. 2. The kinetic problem Oxygen has two unpaired electrons in its ground state and forms therefore a triplet state. The overwhelming majority of organic molecules (such as glucose or n-hexane) have all electrons paired and occur therefore in the singlet state. The products of oxidation of organic molecules, CO 2 and H 2 O, are also in singlet states. According to the so-called Wigner-rule, processes in which the spin-state changes are « spin-forbidden » , that is, they have a large kinetic barrier. The solution of the problem is binding of O 2 to a transition metal complex. In transition metal complexes, spin-state changes are less inhibited due to the spin-orbit coupling. The oxygen-bound metal complex can therefore transit from a triplet state to a singlet state, and then react with an organic substrate which has also a singlet ground-state.

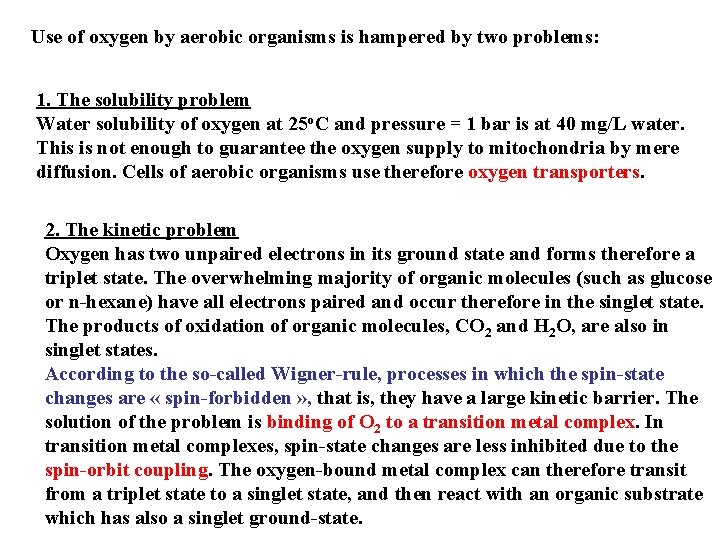
Molecular orbital level diagram for O 2: 3 Sg- state 2 p 2 p 2 s 2 s O O 2 O

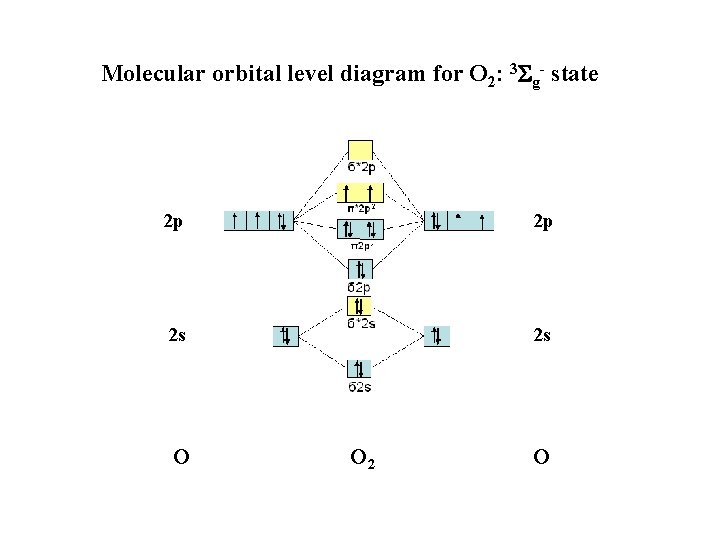
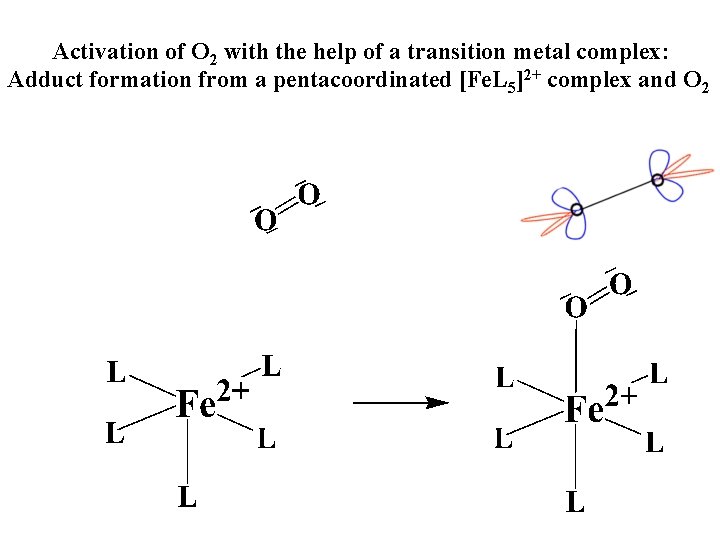
Activation of O 2 with the help of a transition metal complex: Adduct formation from a pentacoordinated [Fe. L 5]2+ complex and O 2

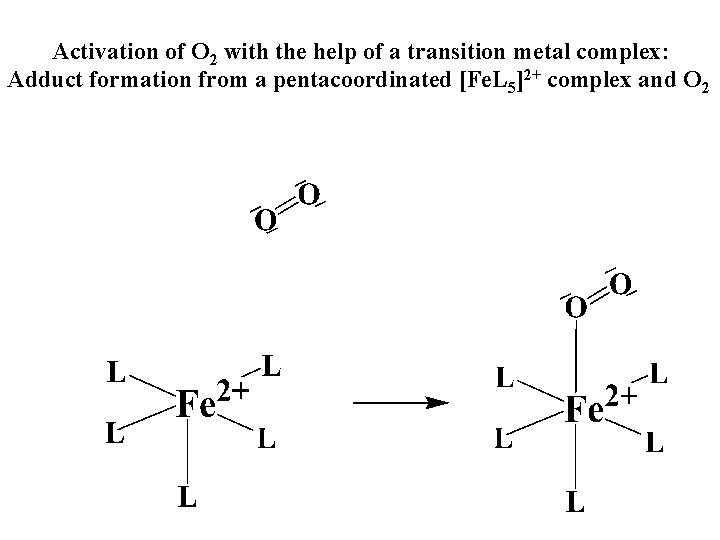
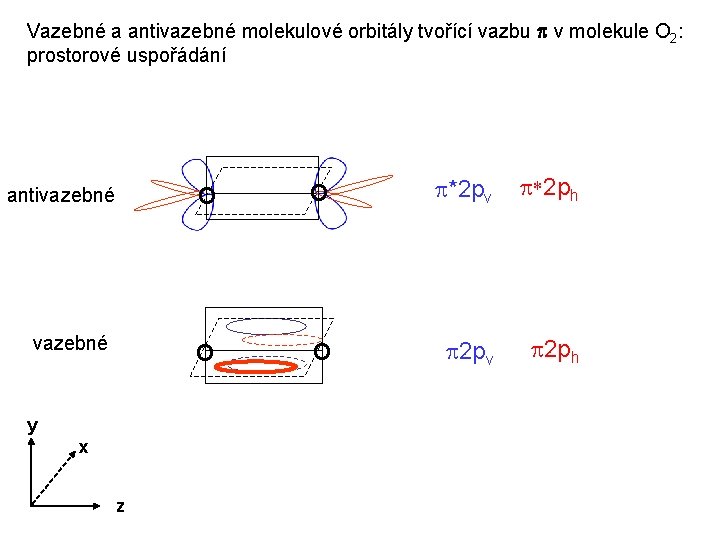
Vazebné a antivazebné molekulové orbitály tvořené atomovými orbitály 2 p v molekule O 2 antivazebné s*2 p p*2 ph xz p*2 pv yz p 2 ph vazebné y x xz p 2 pv yz z Index h = horizontální Index v´= vertikální s 2 p

Vazebné a antivazebné molekulové orbitály tvořící vazbu p v molekule O 2: prostorové uspořádání antivazebné y x z O O p*2 pv p*2 ph O O p 2 pv p 2 ph

Activation of O 2 with the help of a transition metal complex: Adduct formation from a pentacoordinated [Fe. L 5]2+ complex and O 2

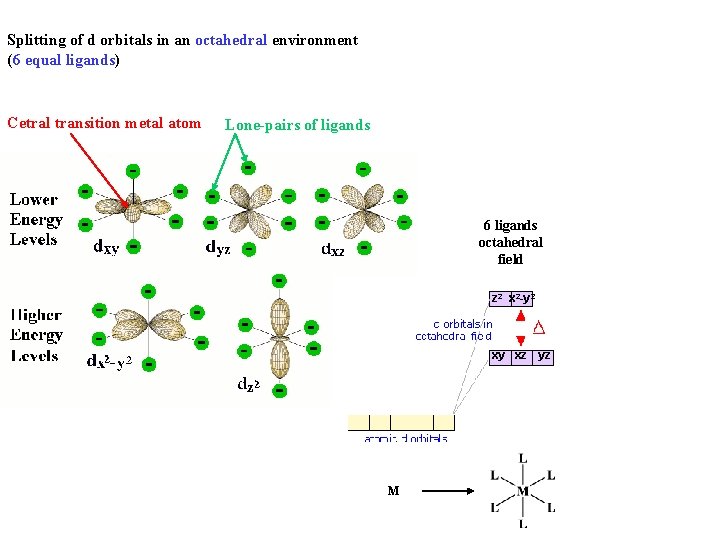
Splitting of d orbitals in an octahedral environment (6 equal ligands) Cetral transition metal atom Lone-pairs of ligands 6 ligands octahedral field z 2 x 2 -y 2 xy xz yz M

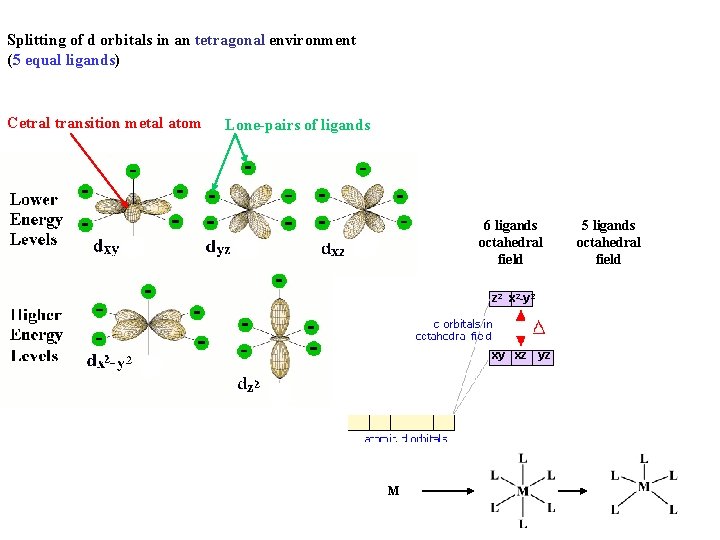
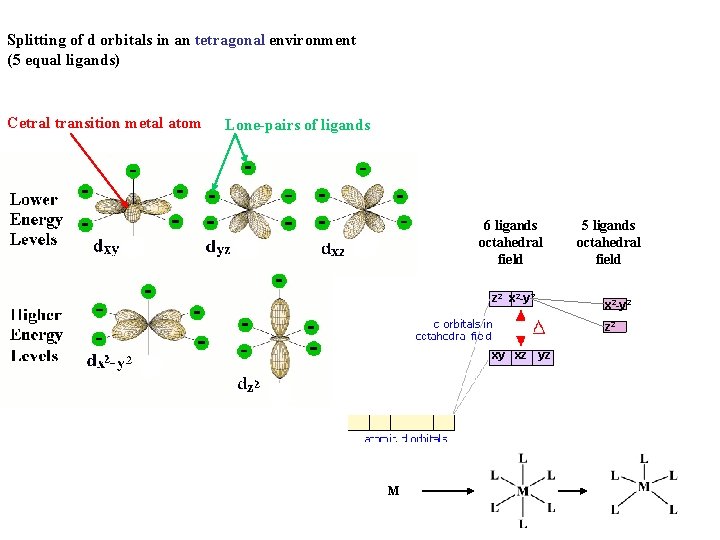
Splitting of d orbitals in an tetragonal environment (5 equal ligands) Cetral transition metal atom Lone-pairs of ligands 6 ligands octahedral field z 2 x 2 -y 2 xy xz yz M 5 ligands octahedral field

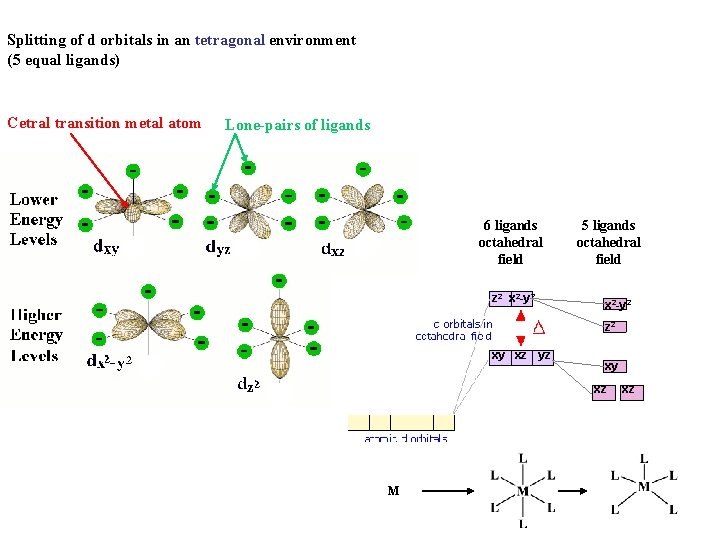
Splitting of d orbitals in an tetragonal environment (5 equal ligands) Cetral transition metal atom Lone-pairs of ligands 6 ligands octahedral field z 2 x 2 -y 2 5 ligands octahedral field x 2 -y 2 z 2 xy xz yz M

Splitting of d orbitals in an tetragonal environment (5 equal ligands) Cetral transition metal atom Lone-pairs of ligands 6 ligands octahedral field z 2 x 2 -y 2 5 ligands octahedral field x 2 -y 2 z 2 xy xz yz xy xz M xz
![3 O 2 1L 5 Fe 3L 5 Fe O 2 spinallowed n 3 O 2 + 1[L 5 Fe] 3[L 5 Fe. O 2] spin-allowed: n°](https://slidetodoc.com/presentation_image/1f1d9308c47edebf9c08aecd8e739791/image-13.jpg)
3 O 2 + 1[L 5 Fe] 3[L 5 Fe. O 2] spin-allowed: n° of unpaired electrons unchanged (only the two unpaired valence electrons shown)
![3 O 2 1L 5 Fe 3L 5 Fe O 2 spinallowed n 3 O 2 + 1[L 5 Fe] 3[L 5 Fe. O 2] spin-allowed: n°](https://slidetodoc.com/presentation_image/1f1d9308c47edebf9c08aecd8e739791/image-14.jpg)
3 O 2 + 1[L 5 Fe] 3[L 5 Fe. O 2] spin-allowed: n° of unpaired electrons unchanged One of the p* orbitals of O 2 overlaps with the dz 2 orbital of Fe and forms a bond; the other p* orbital is non -bonding (only the two unpaired valence electrons shown)
![3 O 2 1L 5 Fe 3L 5 Fe O 2 spinallowed n 3 O 2 + 1[L 5 Fe] 3[L 5 Fe. O 2] spin-allowed: n°](https://slidetodoc.com/presentation_image/1f1d9308c47edebf9c08aecd8e739791/image-15.jpg)
3 O 2 + 1[L 5 Fe] 3[L 5 Fe. O 2] spin-allowed: n° of unpaired electrons unchanged One of the p* orbitals of O 2 overlaps with the dz 2 orbital of Fe and forms a bond; the other p* orbital is non -bonding spin inversion (only the two unpaired valence electrons shown) process spin-forbidden but rendered possible by spin-orbit coupling

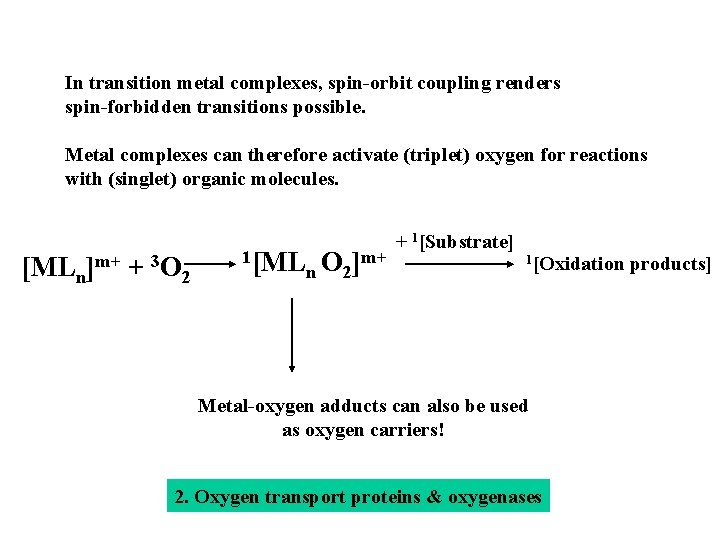
In transition metal complexes, spin-orbit coupling renders spin-forbidden transitions possible. Metal complexes can therefore activate (triplet) oxygen for reactions with (singlet) organic molecules. [MLn]m+ + 3 O 2 1[ML m+ O ] n 2 + 1[Substrate] 1[Oxidation products] Metal-oxygen adducts can also be used as oxygen carriers! 2. Oxygen transport proteins & oxygenases

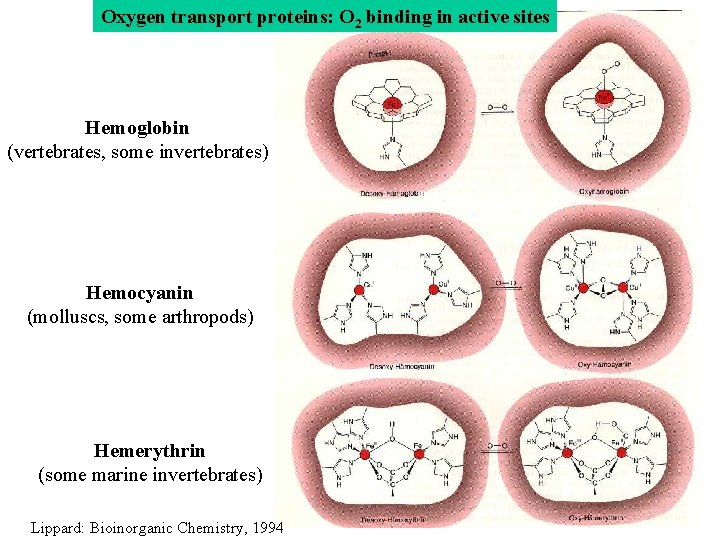
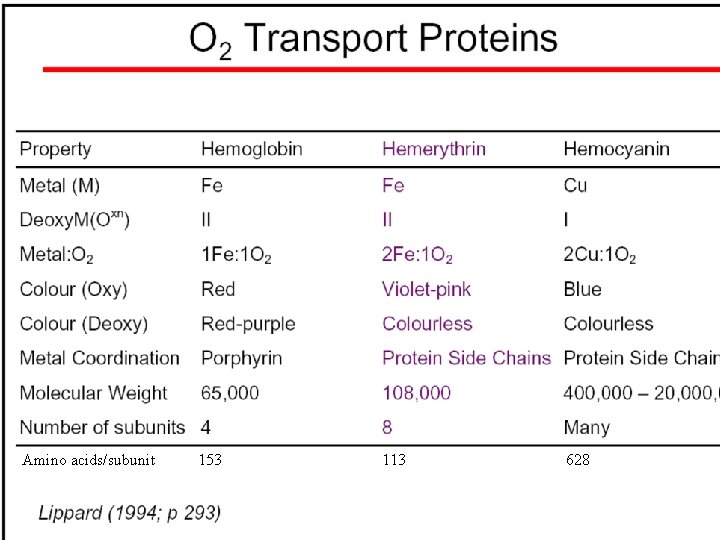
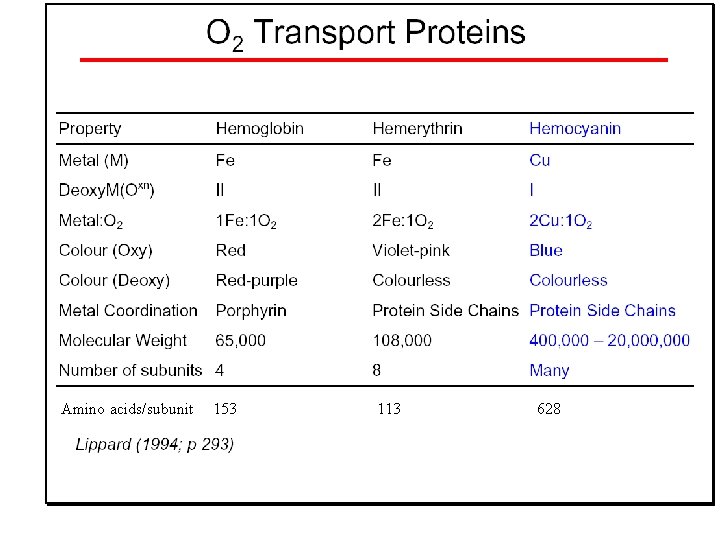
Oxygen transport proteins: O 2 binding in active sites Hemoglobin (vertebrates, some invertebrates) Hemocyanin (molluscs, some arthropods) Hemerythrin (some marine invertebrates) Lippard: Bioinorganic Chemistry, 1994

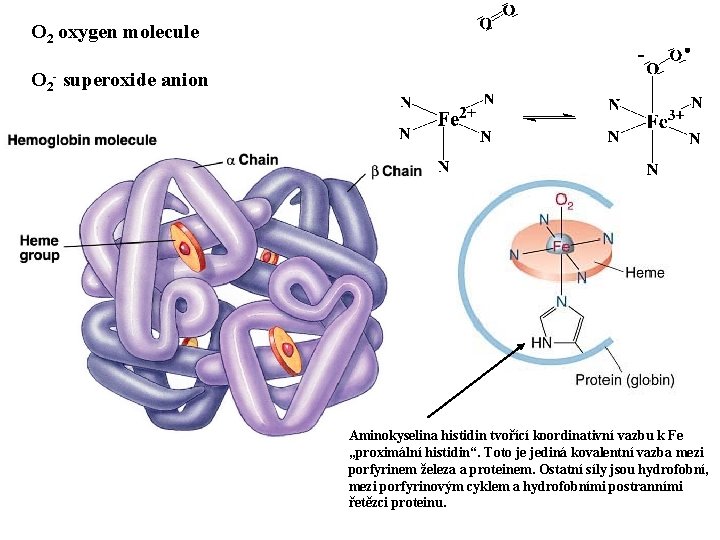
O 2 oxygen molecule O 2 - superoxide anion Aminokyselina histidin tvořící koordinativní vazbu k Fe „proximální histidin“. Toto je jediná kovalentní vazba mezi porfyrinem železa a proteinem. Ostatní síly jsou hydrofobní, mezi porfyrinovým cyklem a hydrofobními postranními řetězci proteinu.


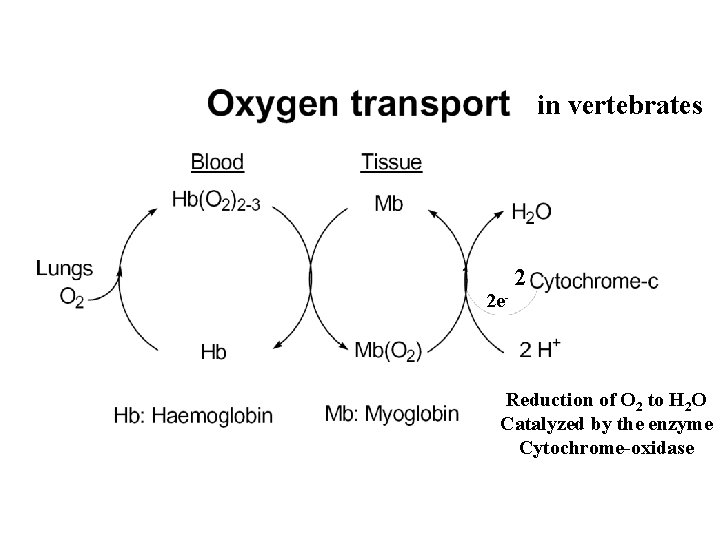
in vertebrates 2 e- 2 Reduction of O 2 to H 2 O Catalyzed by the enzyme Cytochrome-oxidase

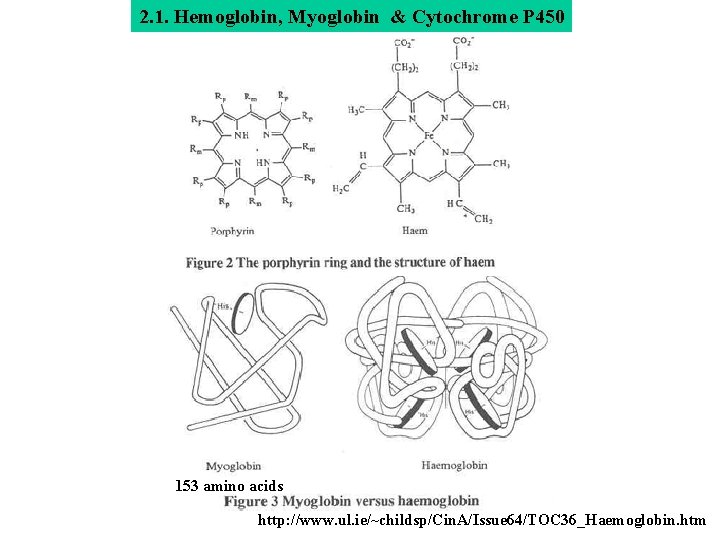
2. 1. Hemoglobin, Myoglobin & Cytochrome P 450 153 amino acids http: //www. ul. ie/~childsp/Cin. A/Issue 64/TOC 36_Haemoglobin. htm

Vazba myoglobinu (Mb) na kyslík Mb + O 2 Mb. O 2 Cvičení 1: definujte rovnovážnou konstantu pro zpětnou reakci (tzv. disociační konstantu, Kd) Cvičení 2: definujte saturaci vazebných míst, Y, definovanou rovnicí dole, pomocí Kd a [O 2] jako proměnných. Nahraďte ve vzorcích pro Kd a pro Y koncentraci [O 2] parciálním tlakem p(O 2).

Cvičení 3 Vypočtěte křivku frakční saturace kyslíku na myoglobinu. Disociační konstanta komplexu Mb. O 2 je, při 37 °C, p. H = 7 a p = 760 Torr, Kd = 2. 8 Torr. p(O 2) [Torr] 0. 5 1 2 3 5 10 20 30 40 50 60 70 80 90 Y [%] Cvičení 4: Jaký význam má směrnice saturační křivky v bodě p(O 2) = 0? Znázorněte graficky závislost d. Y/dp(O 2) na p(O 2)

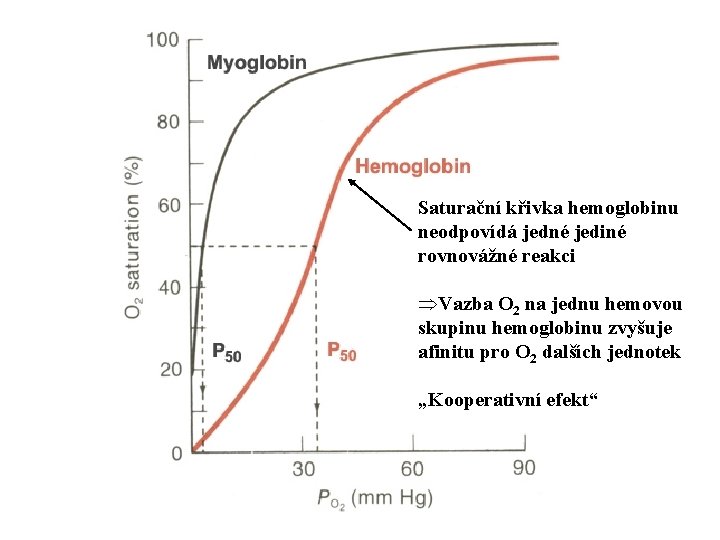
Saturační křivka hemoglobinu neodpovídá jedné jediné rovnovážné reakci ÞVazba O 2 na jednu hemovou skupinu hemoglobinu zvyšuje afinitu pro O 2 dalších jednotek „Kooperativní efekt“

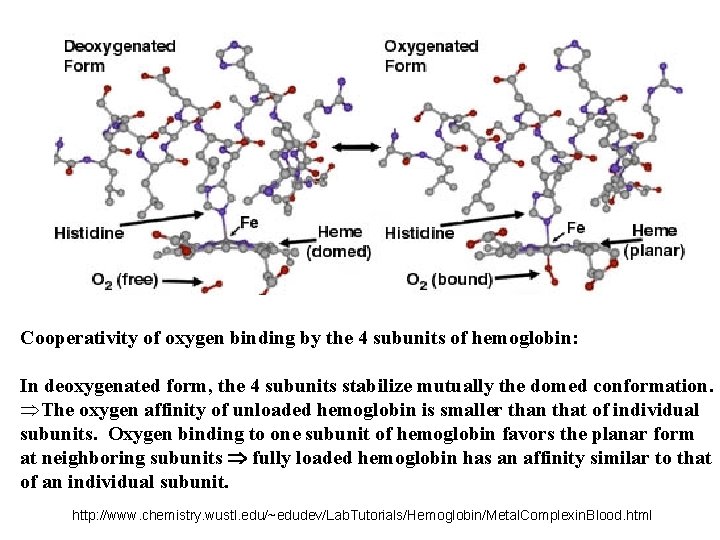
Cooperativity of oxygen binding by the 4 subunits of hemoglobin: In deoxygenated form, the 4 subunits stabilize mutually the domed conformation. ÞThe oxygen affinity of unloaded hemoglobin is smaller than that of individual subunits. Oxygen binding to one subunit of hemoglobin favors the planar form at neighboring subunits fully loaded hemoglobin has an affinity similar to that of an individual subunit. http: //www. chemistry. wustl. edu/~edudev/Lab. Tutorials/Hemoglobin/Metal. Complexin. Blood. html

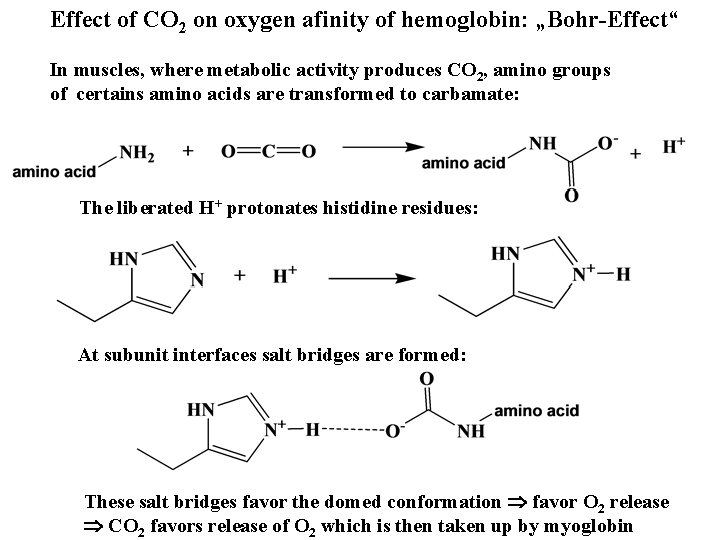
Effect of CO 2 on oxygen afinity of hemoglobin: „Bohr-Effect“ In muscles, where metabolic activity produces CO 2, amino groups of certains amino acids are transformed to carbamate: The liberated H+ protonates histidine residues: At subunit interfaces salt bridges are formed: These salt bridges favor the domed conformation favor O 2 release CO 2 favors release of O 2 which is then taken up by myoglobin

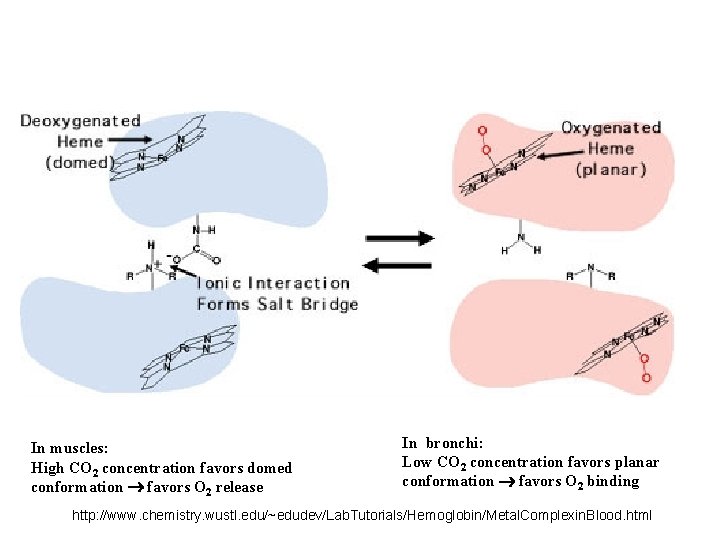
In muscles: High CO 2 concentration favors domed conformation favors O 2 release In bronchi: Low CO 2 concentration favors planar conformation favors O 2 binding http: //www. chemistry. wustl. edu/~edudev/Lab. Tutorials/Hemoglobin/Metal. Complexin. Blood. html

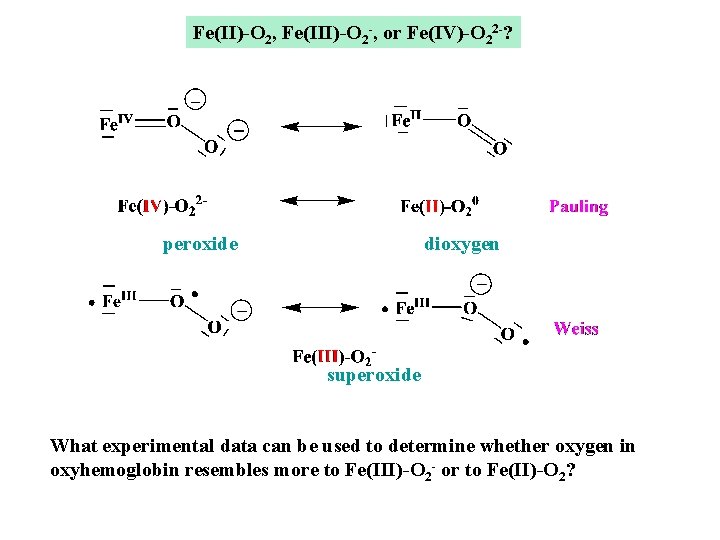
Fe(II)-O 2, Fe(III)-O 2 -, or Fe(IV)-O 22 -? peroxide dioxygen superoxide What experimental data can be used to determine whether oxygen in oxyhemoglobin resembles more to Fe(III)-O 2 - or to Fe(II)-O 2?
![Stretching frequencies and bond lengths in dioxygen species Species A n OO cm1 d Stretching frequencies and bond lengths in dioxygen species Species [A] n. O-O [cm-1] d](https://slidetodoc.com/presentation_image/1f1d9308c47edebf9c08aecd8e739791/image-29.jpg)
Stretching frequencies and bond lengths in dioxygen species Species [A] n. O-O [cm-1] d O-O O 2 + 1905 1. 12 O 2 1580 1. 21 O 2 - 1097 1. 33 O 22 - 802 1. 49 M-O 2 - 1100 -1150 1. 24 -1. 31 M- O 22 - 800 -900 1. 35 -1. 50 1105 1. 22 Mb-O 2 Oxymyoglobin resembles Fe. III-O 2 -

F 5351 Metalloproteins reacting with oxygen 1. Why do aerobic organisms need metalloproteins? 2. Oxygen transport proteins & Oxygenases 2. 1. Hemoglobin, Myoglobin & Cytochrome P 450 2. 2. Hemerythrin & Methane monooxygenase 2. 3. Hemocyanin & Tyrosinase 3. Conclusion Jiří Kozelka 13. 11. 2014 kozelka. jiri@gmail. com

Hemoproteins: Axial Ligands and Functions From: Cécile Claude, „Enzyme Models of Chloroperoxidase and Catalase“, Inaugural Dissertation, Universität Basel, 2001

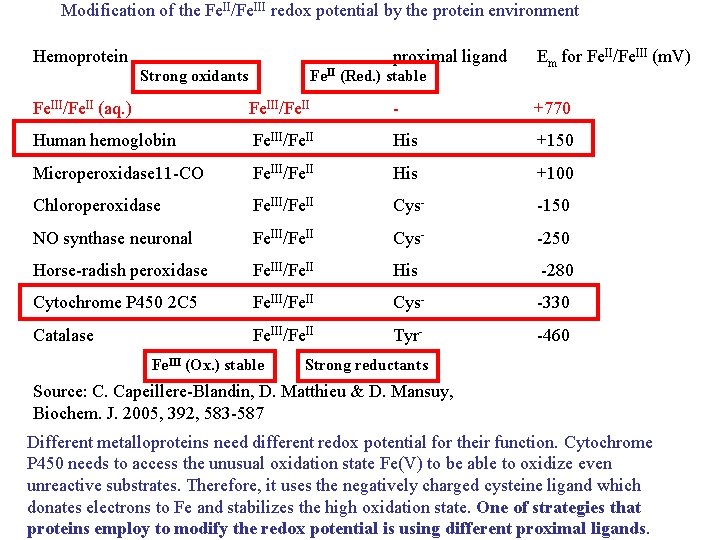
Modification of the Fe. II/Fe. III redox potential by the protein environment Hemoprotein proximal ligand Strong oxidants Fe. II (Red. ) stable Em for Fe. II/Fe. III (m. V) Fe. III/Fe. II (aq. ) Fe. III/Fe. II - +770 Human hemoglobin Fe. III/Fe. II His +150 Microperoxidase 11 -CO Fe. III/Fe. II His +100 Chloroperoxidase Fe. III/Fe. II Cys- -150 NO synthase neuronal Fe. III/Fe. II Cys- -250 Horse-radish peroxidase Fe. III/Fe. II His -280 Cytochrome P 450 2 C 5 Fe. III/Fe. II Cys- -330 Catalase Fe. III/Fe. II Tyr- -460 Fe. III (Ox. ) stable Strong reductants Source: C. Capeillere-Blandin, D. Matthieu & D. Mansuy, Biochem. J. 2005, 392, 583 -587 Different metalloproteins need different redox potential for their function. Cytochrome P 450 needs to access the unusual oxidation state Fe(V) to be able to oxidize even unreactive substrates. Therefore, it uses the negatively charged cysteine ligand which donates electrons to Fe and stabilizes the high oxidation state. One of strategies that proteins employ to modify the redox potential is using different proximal ligands.

Examples of Cytochrome P 450 substrates Hydroxylation at: -aliphatic carbons -aromatic carbons -double bonds -heteroatoms local anesthetic steroid hormone carcinogen from fungi antibiotic Alkaloid from Taxus brevifolia, potent anti-cancer drug

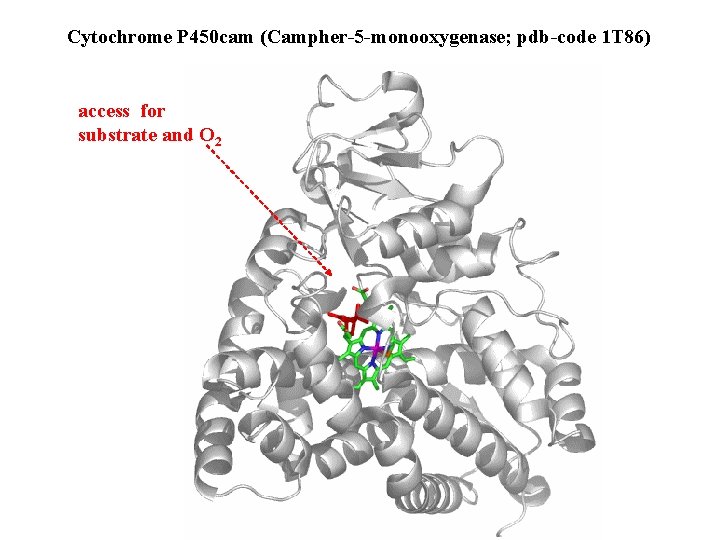
Cytochrome P 450 cam (Campher-5 -monooxygenase; pdb-code 1 T 86) access for substrate and O 2

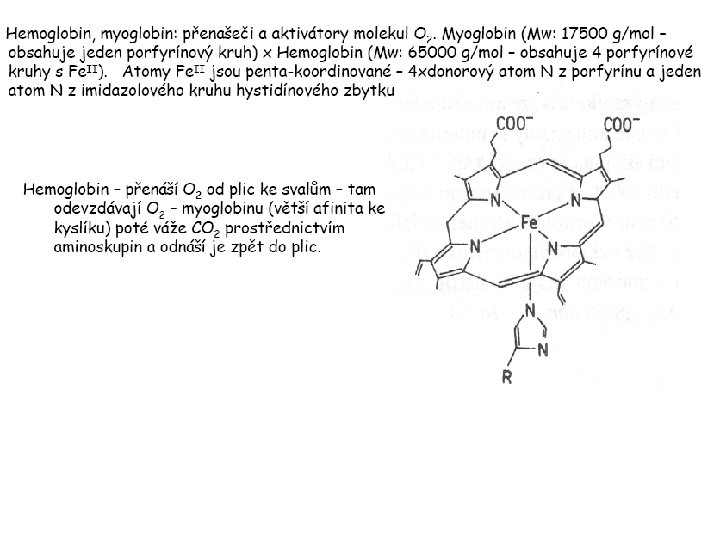
Hlavní dva rozdíly mezi hemoproteiny myoglobin a cytochrom P 450, důležité pro jejich různé funkce: 1. Přístupový kanál vedoucí ke kofaktoru (hemu) je u myoglobinu velmi úzký, nedovoluje přístup větším molekulám než O 2. U cytochromu P 450 je kanál širší a v blízkosti kofaktoru obsahuje místo s vysokou afinitou pro specifické substráty. 2. Distální cystein a okolí kofaktoru snižuje u cytochromu P 450 oxidačněredukční potenciál Fe, takže tento metaloprotein může fungovat jako oxygenáza a Fe v katalytickém cyklu může krátkodobě existovat v oxidačním stupni Fe(V). Tento velmi reaktivní přechodný stav je schopen hydroxylovat i poměrně nereaktivní alifatické atomy uhlíku.

F 5351 Metalloproteins reacting with oxygen 1. Why do aerobic organisms need metalloproteins? 2. Oxygen transport proteins & Oxygenases 2. 1. Hemoglobin, Myoglobin & Cytochrome P 450 2. 2. Hemerythrin & Methane monooxygenase 2. 3. Hemocyanin & Tyrosinase 3. Conclusion Jiří Kozelka 13. 11. 2014 kozelka. jiri@gmail. com

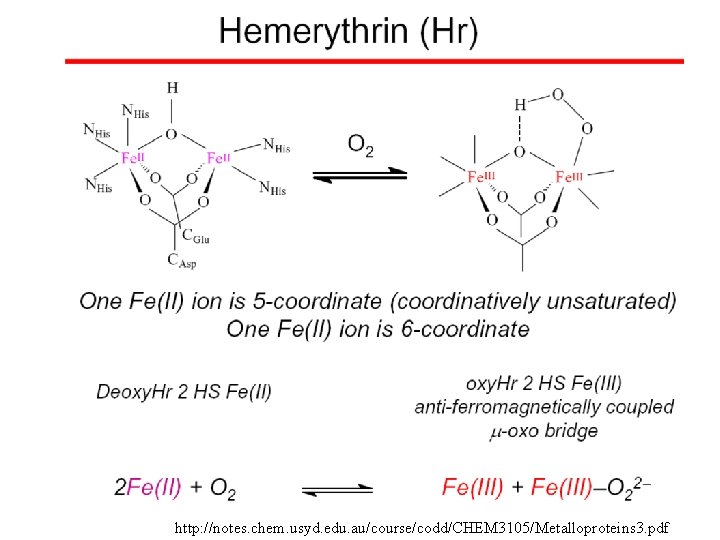
http: //notes. chem. usyd. edu. au/course/codd/CHEM 3105/Metalloproteins 3. pdf

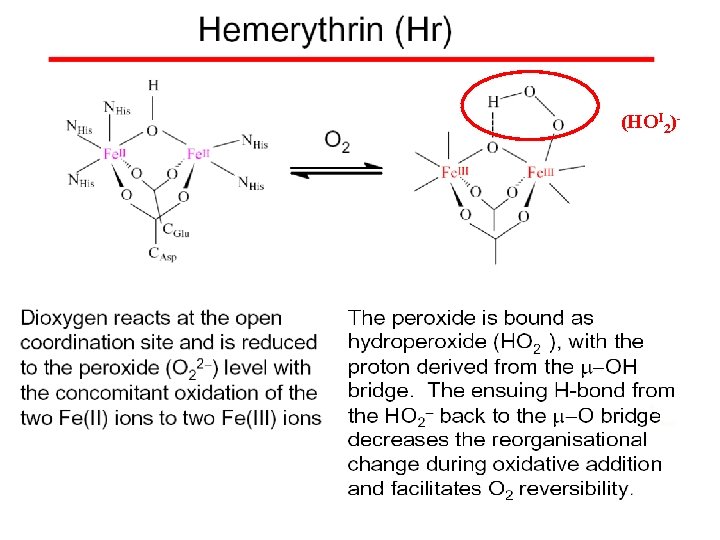
(HOI 2)-

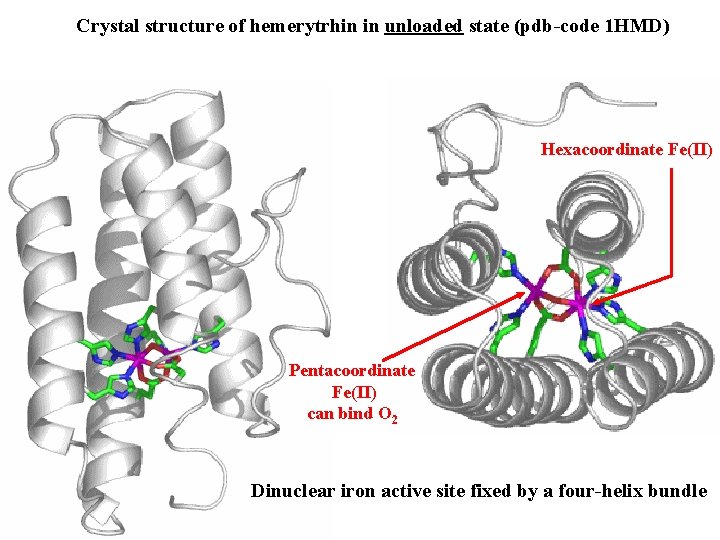
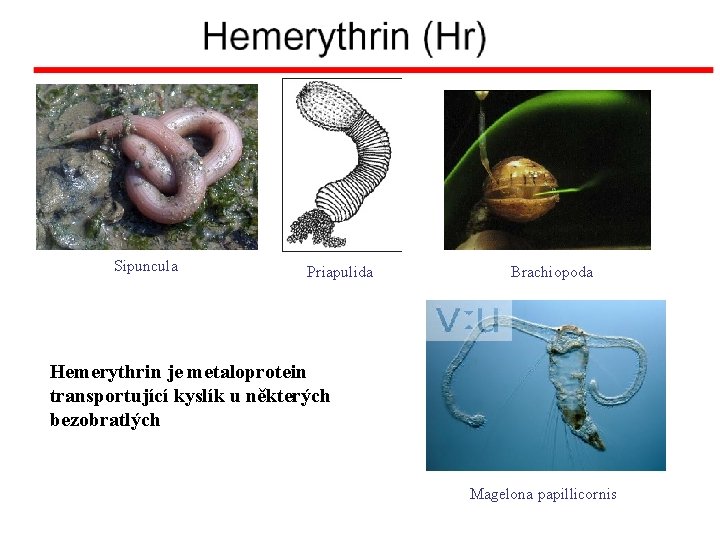
Crystal structure of hemerytrhin in unloaded state (pdb-code 1 HMD) Hexacoordinate Fe(II) Pentacoordinate Fe(II) can bind O 2 Dinuclear iron active site fixed by a four-helix bundle

Amino acids/subunit 153 113 628

Sipuncula Priapulida Brachiopoda Hemerythrin je metaloprotein transportující kyslík u některých bezobratlých Magelona papillicornis

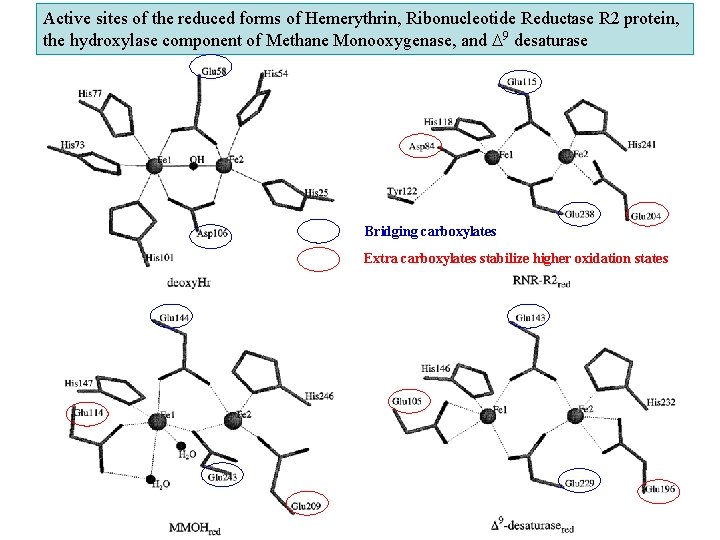
Active sites of the reduced forms of Hemerythrin, Ribonucleotide Reductase R 2 protein, the hydroxylase component of Methane Monooxygenase, and D 9 desaturase Bridging carboxylates Extra carboxylates stabilize higher oxidation states

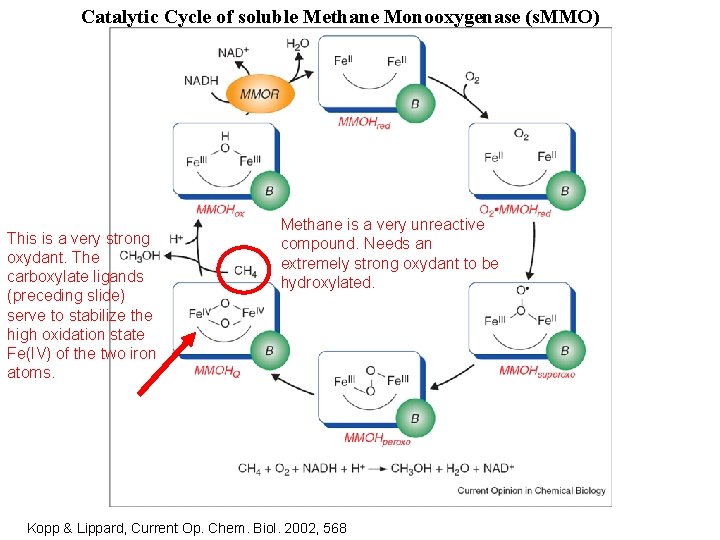
Catalytic Cycle of soluble Methane Monooxygenase (s. MMO) This is a very strong oxydant. The carboxylate ligands (preceding slide) serve to stabilize the high oxidation state Fe(IV) of the two iron atoms. Methane is a very unreactive compound. Needs an extremely strong oxydant to be hydroxylated. Kopp & Lippard, Current Op. Chem. Biol. 2002, 568

F 5351 Metalloproteins reacting with oxygen 1. Why do aerobic organisms need metalloproteins? 2. Oxygen transport proteins & Oxygenases 2. 1. Hemoglobin, Myoglobin & Cytochrome P 450 2. 2. Hemerythrin & Methane monooxygenase 2. 3. Hemocyanin & Tyrosinase 3. Conclusion Jiří Kozelka 13. 11. 2014 kozelka. jiri@gmail. com

Amino acids/subunit 153 113 628

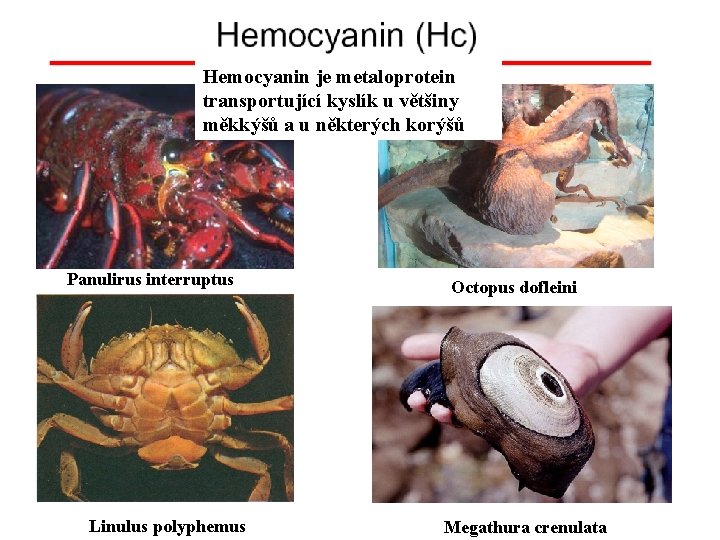
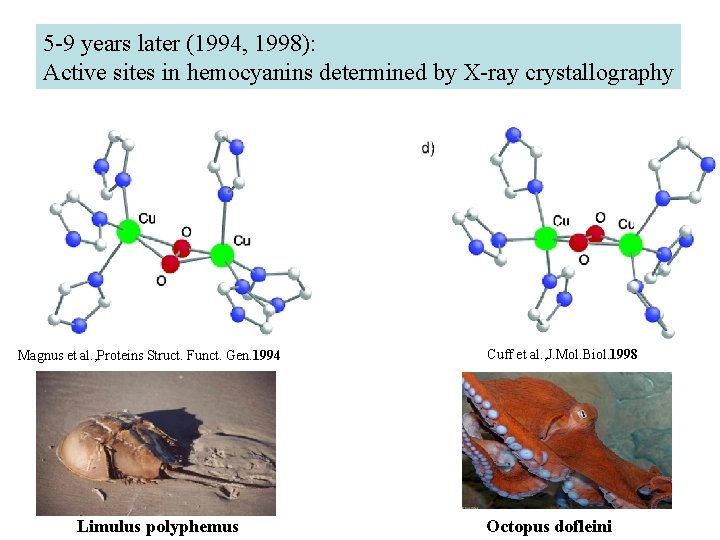
Hemocyanin je metaloprotein transportující kyslík u většiny měkkýšů a u některých korýšů Panulirus interruptus Linulus polyphemus Octopus dofleini Megathura crenulata


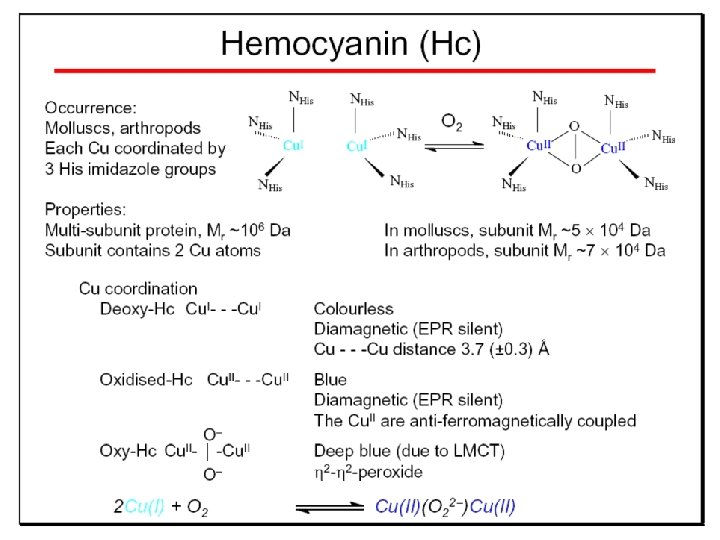
Hemocyanin: History 1878 Leon Federicq: Sur l‘hemocyanine, substance nouvelle de sang de Poulpe (Octopus vulgaris) (Compt. Rend. Acad. Sci. 87, 996 -998) Discovery 1901 M. Henze: Zur Kenntniss des Haemocyanins Z. Physiol. Chem. 33, 370 Hemocyanin contains copper 1940 W. A. Rawlinson, Australian J. Exp. Biol. Med. Sci. 18, 131 Oxy-hemocyanin is diamagnetic

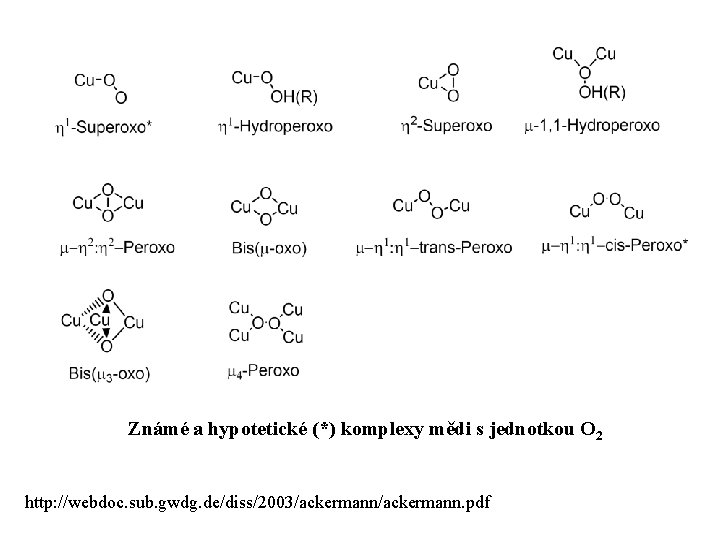
Známé a hypotetické (*) komplexy mědi s jednotkou O 2 http: //webdoc. sub. gwdg. de/diss/2003/ackermann. pdf

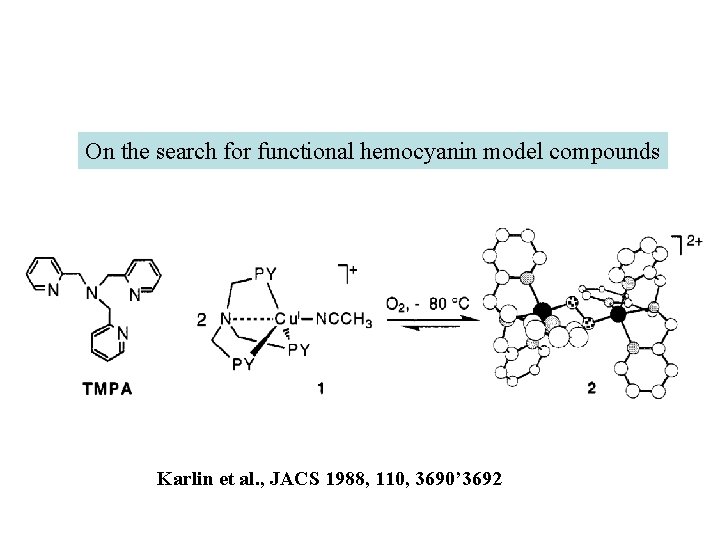
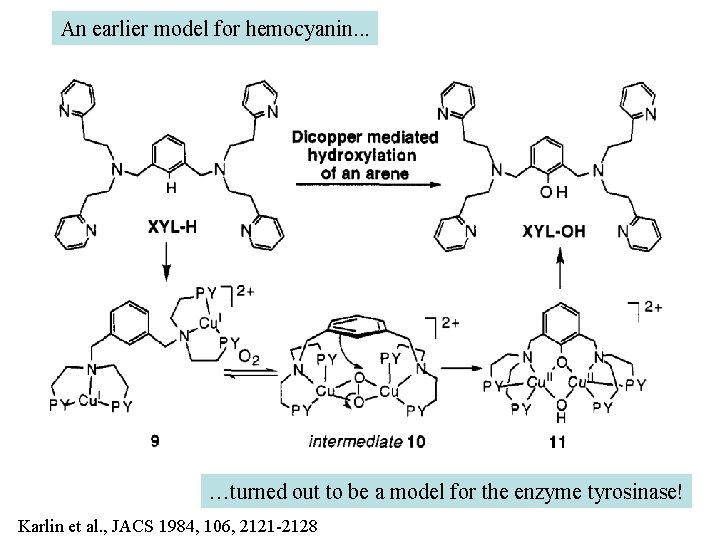
On the search for functional hemocyanin model compounds Karlin et al. , JACS 1988, 110, 3690’ 3692

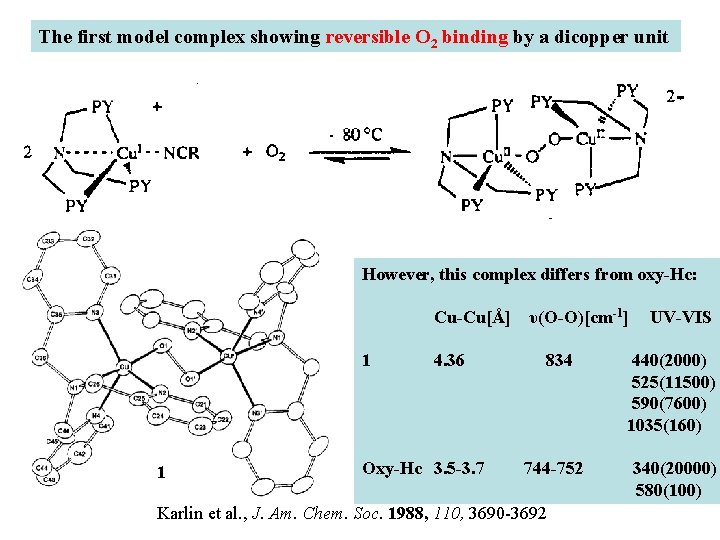
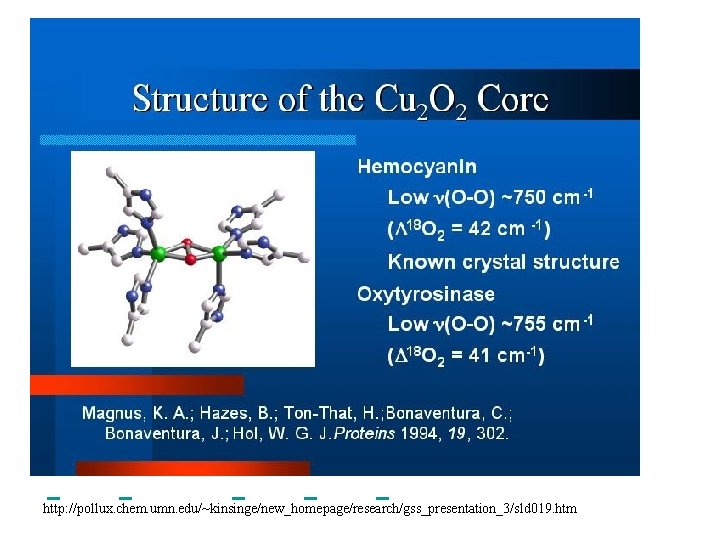
The first model complex showing reversible O 2 binding by a dicopper unit However, this complex differs from oxy-Hc: Cu-Cu[Å] υ(O-O)[cm-1] 1 UV-VIS 4. 36 834 440(2000) 525(11500) 590(7600) 1035(160) Oxy-Hc 3. 5 -3. 7 744 -752 340(20000) 580(100) Karlin et al. , J. Am. Chem. Soc. 1988, 110, 3690 -3692 1

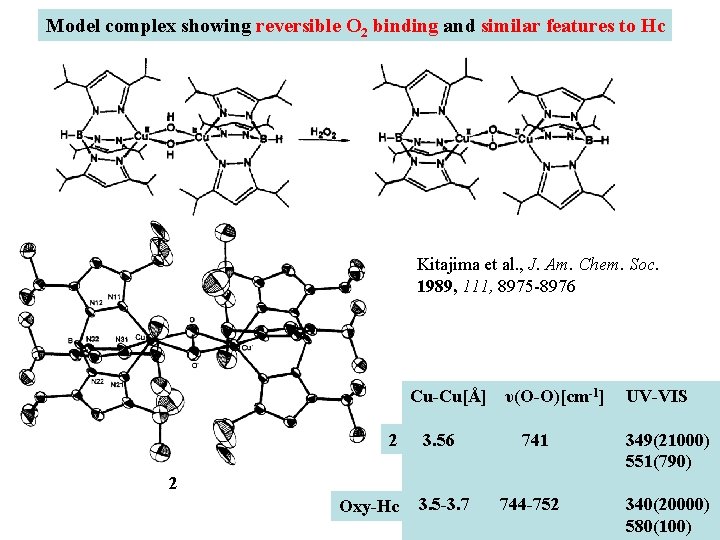
Model complex showing reversible O 2 binding and similar features to Hc Kitajima et al. , J. Am. Chem. Soc. 1989, 111, 8975 -8976 Cu-Cu[Å] υ(O-O)[cm-1] 2 3. 56 741 UV-VIS 349(21000) 551(790) 2 Oxy-Hc 3. 5 -3. 7 744 -752 340(20000) 580(100)
![Functional hemocyanin models tmpa2 Cu 2 O 22 CuHB3 5 i Pr 2 pz32O Functional hemocyanin models [(tmpa)2 Cu 2 O 2]2+ [Cu{HB(3, 5 -i. Pr 2 pz)3}]2(O](https://slidetodoc.com/presentation_image/1f1d9308c47edebf9c08aecd8e739791/image-53.jpg)
Functional hemocyanin models [(tmpa)2 Cu 2 O 2]2+ [Cu{HB(3, 5 -i. Pr 2 pz)3}]2(O 2) Karlin et al. , JACS 1988, 110, 3690’ 3692 Kitajima et al. , JACS 1989, 111, 8975 -8976

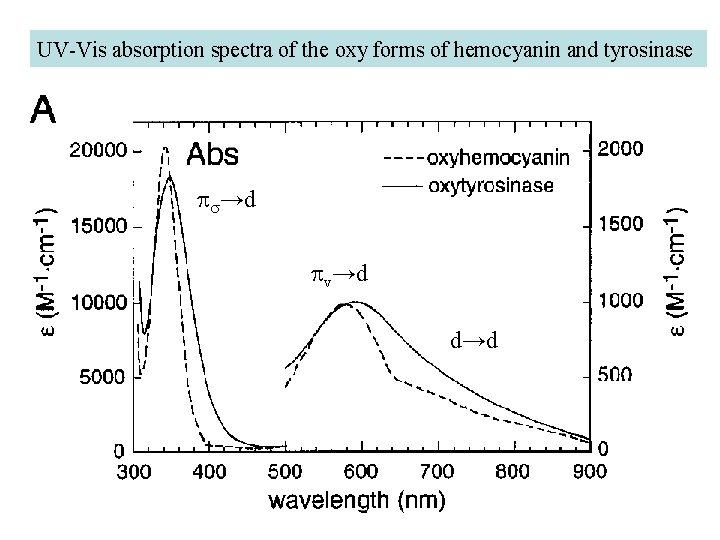
UV-Vis absorption spectra of the oxy forms of hemocyanin and tyrosinase ps→d pv→d d→d

5 -9 years later (1994, 1998): Active sites in hemocyanins determined by X-ray crystallography Magnus et al. , Proteins Struct. Funct. Gen. 1994 Limulus polyphemus Cuff et al. , J. Mol. Biol. 1998 Octopus dofleini

An earlier model for hemocyanin. . . …turned out to be a model for the enzyme tyrosinase! Karlin et al. , JACS 1984, 106, 2121 -2128

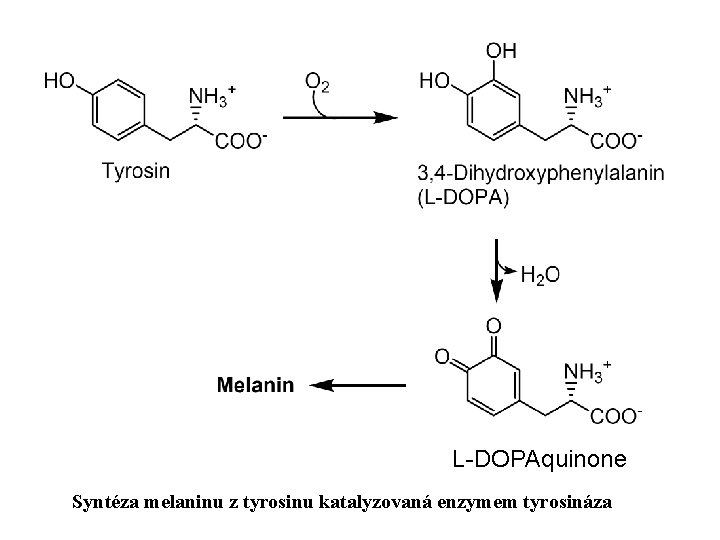
L-DOPAquinone Syntéza melaninu z tyrosinu katalyzovaná enzymem tyrosináza

http: //pollux. chem. umn. edu/~kinsinge/new_homepage/research/gss_presentation_3/sld 019. htm

Tyrosinase versus Hemocyanin The coupled binuclear copper sites in tyrosinase and hemocyanin are very similar. Why is then tyrosinase capable of reacting with substrates while hemocyanin is not? Solomon (Angew. Chem. Int. Ed. Engl. 2001, 40, 4570 -450): Difference in accessibility of the active site Rates of peroxide displacement by azide (measured using UV absorption) at 4°C: Hemocyanins: k £ 0. 04 h-1 Tyrosinase: k = 0. 95 h-1

Hypothesis, 1980: Solomon et al. , JACS 1980, 102, 7339 -7344, p. 7343 Angew. Chem. Int. Ed. 2001, 40, 4570 -4590 Proof, 1998 (J. Biol. Chem. 273, 25889 -25892):

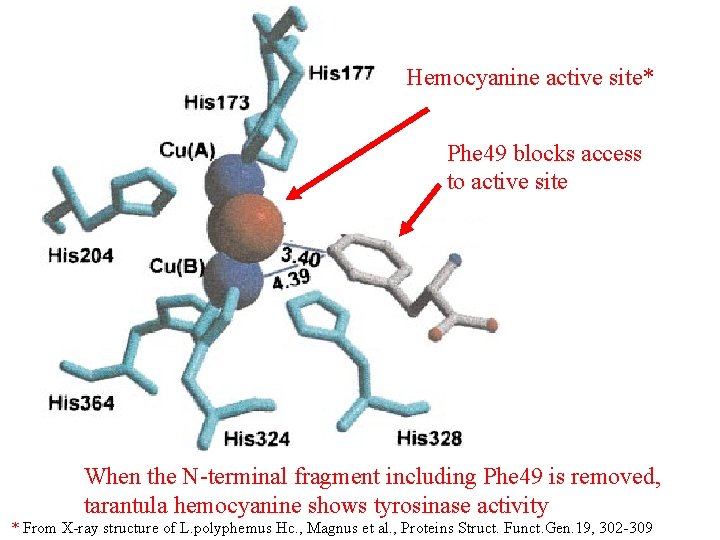
Hemocyanine active site* Phe 49 blocks access to active site When the N-terminal fragment including Phe 49 is removed, tarantula hemocyanine shows tyrosinase activity * From X-ray structure of L. polyphemus Hc. , Magnus et al. , Proteins Struct. Funct. Gen. 19, 302 -309

Conclusions In many cases, metalloproteins use the same or similar active site for different purposes. The strategies to confer a particular activity to a given site include - Allowing/disallowing access of substrates to the active site (including the dynamics of diffusion of substrate/product) -Modifying the electrostatic potential by mutating the amino acids coordinated to the metal or surrounding the binding pocket -Architecture of the binding pocket defines substrate selectivity and affects energy of transition states→governs reaction outcome
 Lithium react with oxygen equation
Lithium react with oxygen equation Magnesium reacting with oxygen
Magnesium reacting with oxygen Copper and oxygen reaction
Copper and oxygen reaction Why why why why
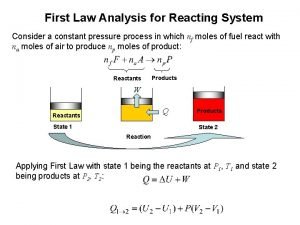
Why why why why Reacting system
Reacting system Magnesium reacting with nitric acid equation
Magnesium reacting with nitric acid equation Reacting masses questions
Reacting masses questions Reacting masses and volumes
Reacting masses and volumes Sodium carbonate and sodium bicarbonate
Sodium carbonate and sodium bicarbonate Alkali metals reacting with water
Alkali metals reacting with water React to direct fire while mounted
React to direct fire while mounted Reacting to indirect fire
Reacting to indirect fire Alkali metals reacting with water
Alkali metals reacting with water Alkali metals reacting with water
Alkali metals reacting with water Don't ask why why why
Don't ask why why why Why do cells need oxygen?
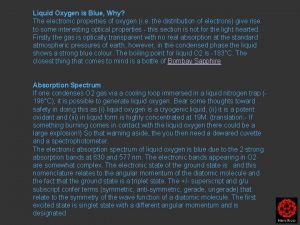
Why do cells need oxygen? Why is liquid oxygen blue
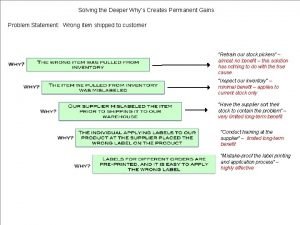
Why is liquid oxygen blue Why-why analysis
Why-why analysis Wh tongue twister
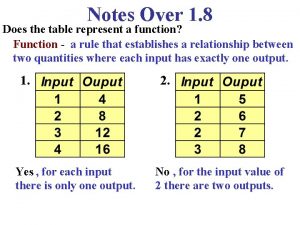
Wh tongue twister Does this table represent a function why or why not
Does this table represent a function why or why not What does a table represent
What does a table represent Why or why not
Why or why not Contoh root cause analysis 5 why
Contoh root cause analysis 5 why Molare masse
Molare masse How many electrons does oxygen have
How many electrons does oxygen have Oxygen deficient
Oxygen deficient Oxygen index
Oxygen index Cecil writes the equation for the reaction of hydrogen
Cecil writes the equation for the reaction of hydrogen Limewater
Limewater Molar mass of sucrose
Molar mass of sucrose Oxygen sag definition
Oxygen sag definition Gas exchange lungs
Gas exchange lungs The respiratory system
The respiratory system Glucose carbon dioxide and water
Glucose carbon dioxide and water Winkler method principle
Winkler method principle Percent by mass
Percent by mass Oxygen content
Oxygen content Early signs of oxygen toxicity
Early signs of oxygen toxicity Oxygen toxicity
Oxygen toxicity Types of oxygen masks and flow rates
Types of oxygen masks and flow rates T piece oxygen delivery
T piece oxygen delivery Saturation normal
Saturation normal Mary catterall mask
Mary catterall mask Reservoir nasal cannula
Reservoir nasal cannula Trach collar oxygen flow rates
Trach collar oxygen flow rates High flow versus low flow oxygen
High flow versus low flow oxygen Oxygen liters per minute chart
Oxygen liters per minute chart Percent of oxygen in the atmosphere
Percent of oxygen in the atmosphere Oxygen administration definition
Oxygen administration definition Electron configuration of oxygen
Electron configuration of oxygen Reactive oxygen species examples
Reactive oxygen species examples How many protons does oxygen have
How many protons does oxygen have C2h4 lewis structure
C2h4 lewis structure How elements form compounds
How elements form compounds Dissolved oxygen in water
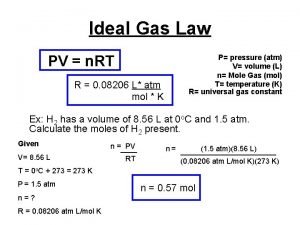
Dissolved oxygen in water Pv=nrt find n
Pv=nrt find n Stroke volume pdhpe
Stroke volume pdhpe Find the number of protons c
Find the number of protons c Aluminum bohr rutherford
Aluminum bohr rutherford Lactic acid + oxygen
Lactic acid + oxygen Oxygen therapy
Oxygen therapy Mass of oxygen in water
Mass of oxygen in water Methane oxygen endothermic or exothermic
Methane oxygen endothermic or exothermic