Extraction of petroleum Extraction of petroleum Extraction of














- Slides: 39

Extraction of petroleum

Extraction of petroleum

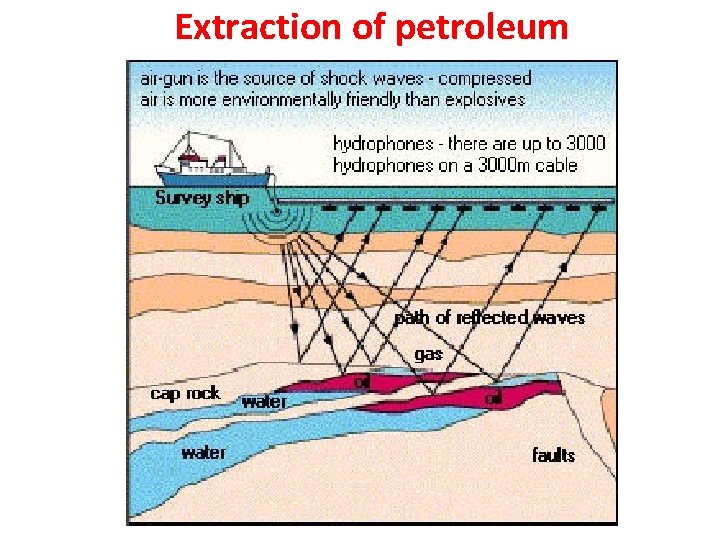
Extraction of petroleum • The extraction of petroleum is the process by which usable petroleum is extracted and removed from the earth. • The first step is Locating the oil field. (Oil field is the region with an abundance of oil reservoirs underground) • Geologists use seismic surveys to search for the oil reservoirs/fields. It involves the use of SHOCK WAVES to get correct information about the geological structures under the ground. • Other instruments such as gravimeters and magnetometers are also sometimes used in the search for petroleum. 3

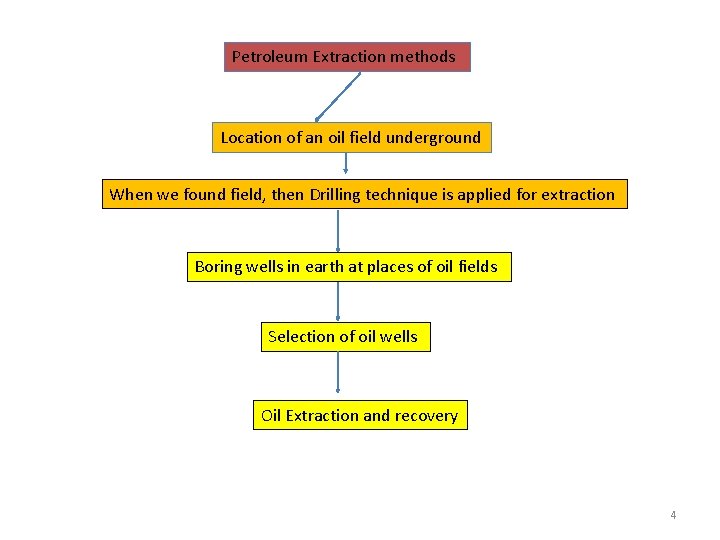
Petroleum Extraction methods Location of an oil field underground When we found field, then Drilling technique is applied for extraction Boring wells in earth at places of oil fields Selection of oil wells Oil Extraction and recovery 4

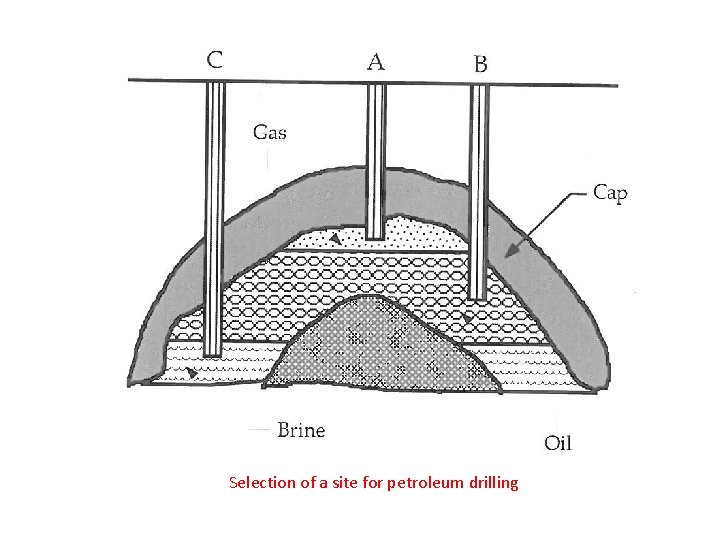
Extraction of petroleum • The extraction of petroleum is normally started with the Drilling wells into the underground reservoirs. It involves primarily Selecting the exact site for drilling the well • Drilling of three wells are shown in figure, all of which are reasonably close to the oil pool, only well B would actually produce oil. • Oil-well is a general term for drilling through which earth surface that is designed to find and produce petroleum. • Drilling is the process by which earth crust is crushed down deeply inside at different depths.

Selection of a site for petroleum drilling

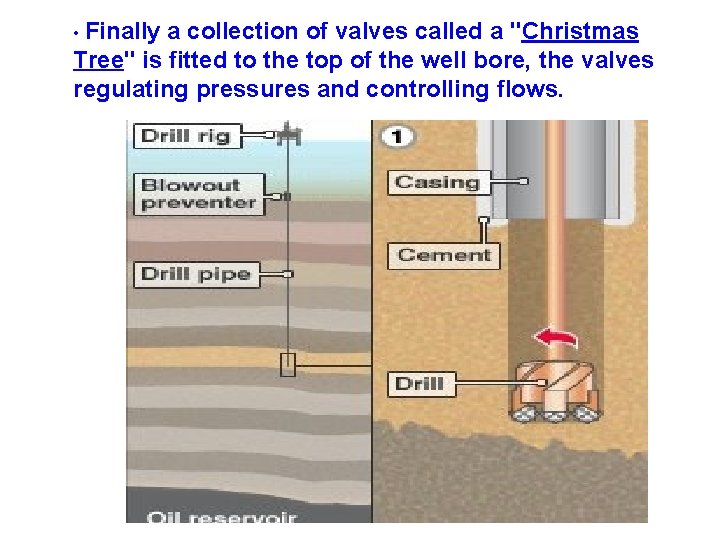
Drilling technique • The oil well is created by drilling a long hole into the earth with an oil rig. It is machine which creates holes in the ground. • A steel pipe (casing) is placed in the hole, to provide structural integrity to the newly drilled well bore. • Holes are then made in the base of the well, by using Drill 7

• Finally a collection of valves called a "Christmas Tree" is fitted to the top of the well bore, the valves regulating pressures and controlling flows.

Drilling technique • The deeper the oil-well, more expensive it becomes because of: • Labor involved in the drilling process • The cost of equipment used • The drilling mud is then examined for traces of oil. Once oil has been found, the drill pipe (drilling rig) is pulled out, leaving the steel pipe (casing) there to protect against collapsing. • After this, the drilling process is said to be COMPLETED. • Completion is the process in which the well is enabled to produce oil or gas. 9

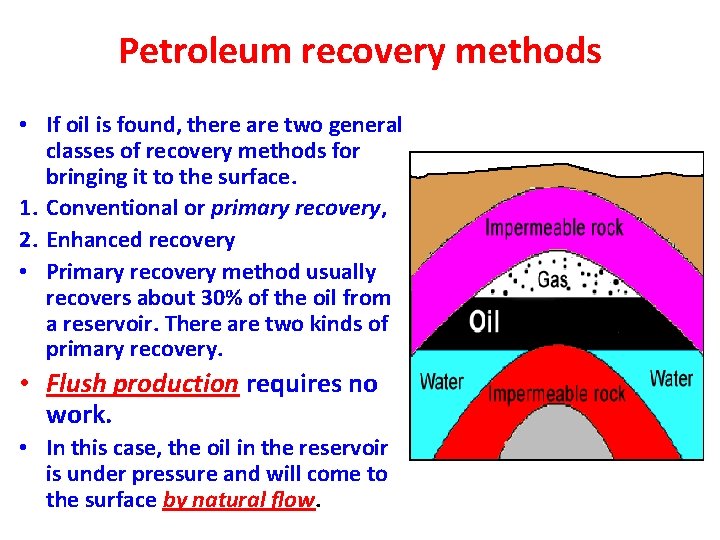
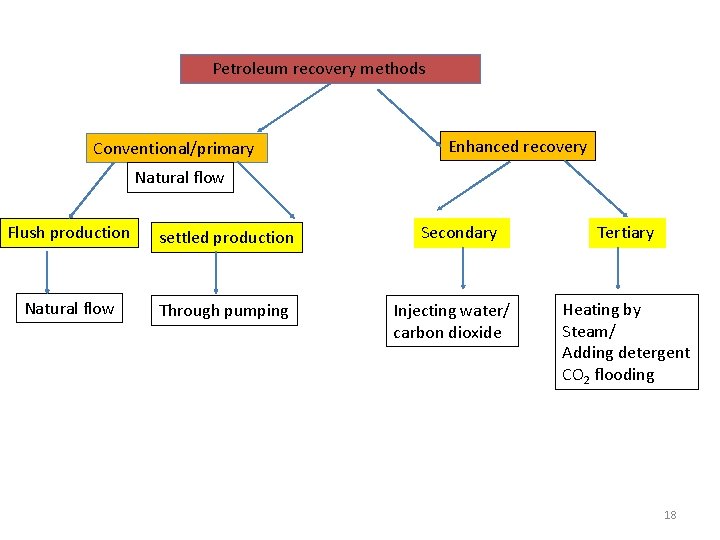
Petroleum recovery methods • If oil is found, there are two general classes of recovery methods for bringing it to the surface. 1. Conventional or primary recovery, 2. Enhanced recovery • Primary recovery method usually recovers about 30% of the oil from a reservoir. There are two kinds of primary recovery. • Flush production requires no work. • In this case, the oil in the reservoir is under pressure and will come to the surface by natural flow.

• The force responsible for the flow of oil may be a water drive, in which water lying under the oil pushes it to the surface, or a gas cap drive, in which a bubble of gas pushing down on the oil forces it to the surface. • Settled production occurs when oil has to be pumped from the reservoir by using PUMPS.

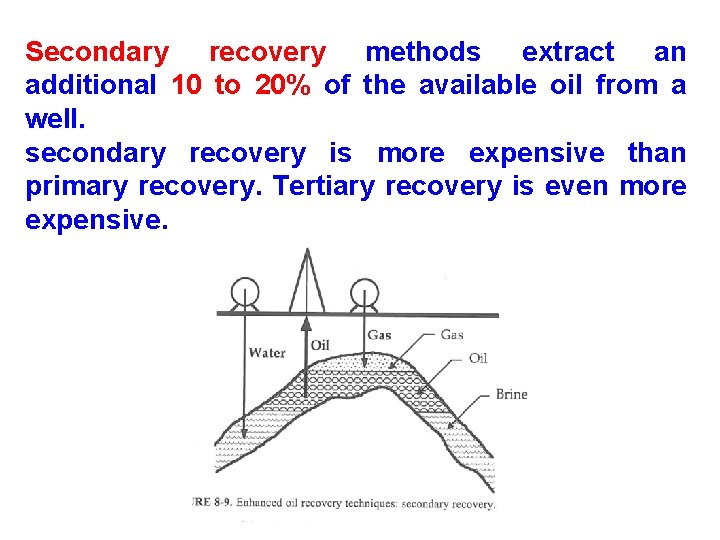
Enhanced Recovery Method • It is used when it is no longer possible to pump the oil with conventional techniques. Enhanced oil recovery techniques are sometimes divided into 1. secondary recovery and 2. tertiary recovery method. • Secondary recovery techniques increase the reservoir's pressure by water injection, natural gas reinjection or injection of air/carbon dioxide gas into the bottom of an active well, reducing the overall density (viscosity) of fluid in the wellbore. 12

Secondary recovery methods extract an additional 10 to 20% of the available oil from a well. secondary recovery is more expensive than primary recovery. Tertiary recovery is even more expensive.

Tertiary oil recovery methods • In general, it is usually necessary to decrease the viscosity of the oil to achieve further recovery. • Since the viscosity of any liquid drops as its temperature increases, tertiary recovery often involves heating the oil underground, such as by injecting steam into the wells. • Thermally enhanced oil recovery methods (TEOR) are tertiary recovery techniques that heat the oil, thus reducing its viscosity and making it easier to extract. • Steam injection is the most common form of TEOR. 14

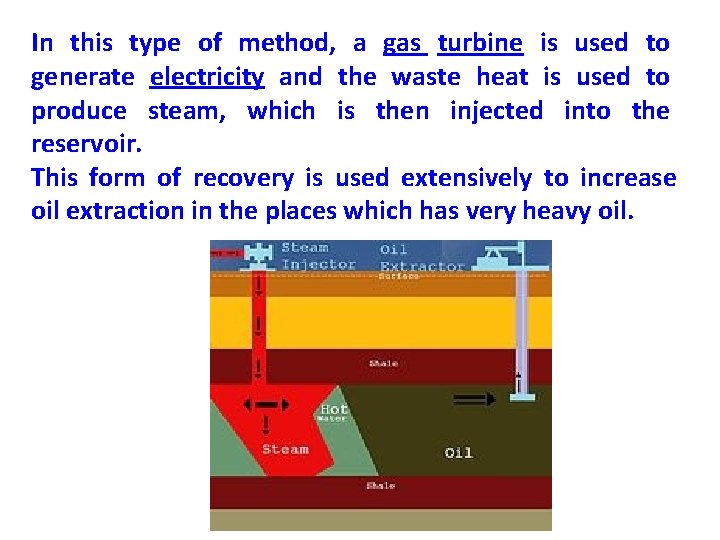
In this type of method, a gas turbine is used to generate electricity and the waste heat is used to produce steam, which is then injected into the reservoir. This form of recovery is used extensively to increase oil extraction in the places which has very heavy oil.

Tertiary oil recovery methods • Tertiary recovery allows another 5% to 15% of the reservoir's oil to be recovered. • Occasionally, surfactants (detergents) are also injected to change the surface tension between the water and oil in the reservoir, mobilizing oil which would otherwise remain in the reservoir as residual oil. • Another method to reduce viscosity is carbon dioxide flooding. 16

• Tertiary recovery begins when secondary oil recovery isn't enough to continue adequate extraction, but only when the oil can still be extracted profitably. • This depends on the cost of the extraction method and the current price of crude oil. • When prices are high, previously unprofitable wells are brought back into use and when they are low, further extraction is stopped.

Petroleum recovery methods Conventional/primary Enhanced recovery Natural flow Flush production settled production Natural flow Through pumping Secondary Injecting water/ carbon dioxide Tertiary Heating by Steam/ Adding detergent CO 2 flooding 18

When will crude oil run out? It is difficult to say when crude oil will run out because no-one knows exactly how much oil there is left in the world. There are over 1 trillion (1 million) barrels of crude oil in proven oil reserves. At current rates, this will last about 44 years, but the amount of oil used in the world increases each year. Some scientists believe there is a lot more oil still to find, but others think that most oil has already been discovered. 19

Chemical composition of crude oil • Main composition • Hydrocarbons – Paraffins – Cycloparaffins – Aromatics – Other hydrocarbons • Non-hydrocarbons – Sulfur compounds – Nitrogen compounds – Trace metals 20

Crude oil: a brief history The modern oil industry began in the mid-19 th century when the original requirement was to produce kerosene from crude oil as a cheaper and better source of light. Gasoline was a by-product in kerosene production, and was initially used as a solvent. The development of the internal combustion engine and gasoline-powered cars in the late 19 th century led to the production of gasoline and 21 diesel fuels.

• The evolution of the airplane created an initial need for high-octane aviation gasoline and then for jet fuel, a sophisticated form of the original product, kerosene. • This led to a great increase in the demand for crude oil, which has continued to this day.

BASICS OF CRUDE OIL • Crude oils are complex mixtures containing many different hydrocarbon compounds that vary in appearance and composition from one oil field to another. • Crude oils range in consistency from water to tarlike solids, and in color from clear to black. • An "average" crude oil contains about 84% carbon, 14% hydrogen, 1%-3% sulfur, and less than 1% each of nitrogen, oxygen, metals, and salts. • Crude oils are generally classified as paraffinic, naphthenic, or aromatic, based on the predominant proportion of similar hydrocarbon molecules. • Mixed-base crudes have varying amounts of each type of hydrocarbon. 23

BASICS OF CRUDE OIL • Crude oils are also defined in terms of API (American Petroleum Institute) gravity. • The higher the API gravity, the lighter the crude. For example, light crude oils have high API gravities and low specific gravities. • Crude oils with low carbon, high hydrogen, and high API gravity are usually rich in paraffins and tend to yield greater proportions of gasoline and light petroleum products. • Crude oils with high carbon, low hydrogen, and low API gravities are usually rich in aromatics. • Crude oils that contain appreciable quantities of hydrogen sulfide or other reactive sulfur compounds are called "sour. " Those with less sulfur are called "sweet. " 24

BASICS OF HYDROCARBON CHEMISTRY • Crude oil is a mixture of hydrocarbon molecules, which are organic compounds of carbon and hydrogen atoms that may include from one to 60 carbon atoms. • The properties of hydrocarbons depend on the number and arrangement of the carbon and hydrogen atoms in the molecules. • The simplest hydrocarbon molecule is one carbon atom linked with four hydrogen atoms: methane. All other variations of petroleum hydrocarbons evolve from this molecule. Hydrocarbons containing up to four carbon atoms are usually gases, those with 5 to 19 carbon atoms are usually liquids, and those with 20 or more are solids. 25

• The refining process uses chemicals, catalysts, heat, and pressure to separate and combine the basic types of hydrocarbon molecules naturally found in crude oil into groups of similar molecules. • The refining process also rearranges their structures and bonding patterns into different hydrocarbon molecules and compounds. • Therefore it is the type of hydrocarbon (paraffinic, naphthenic, or aromatic) rather than its specific chemical compounds that is significant in the refining process.

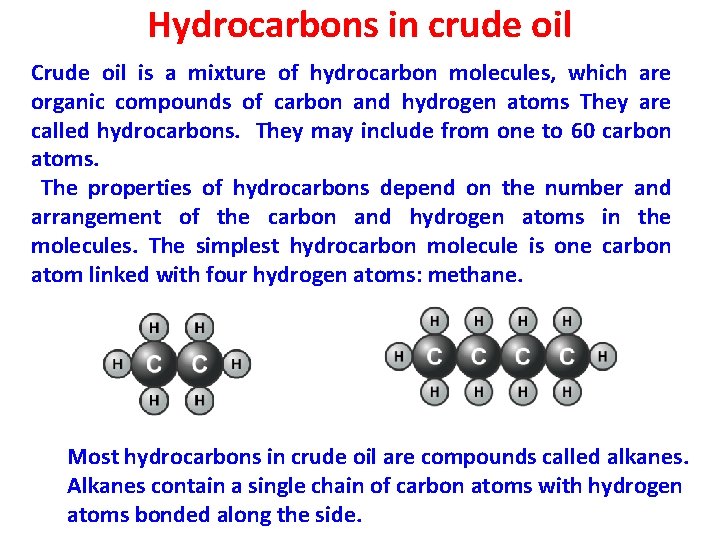
Hydrocarbons in crude oil Crude oil is a mixture of hydrocarbon molecules, which are organic compounds of carbon and hydrogen atoms They are called hydrocarbons. They may include from one to 60 carbon atoms. The properties of hydrocarbons depend on the number and arrangement of the carbon and hydrogen atoms in the molecules. The simplest hydrocarbon molecule is one carbon atom linked with four hydrogen atoms: methane. Most hydrocarbons in crude oil are compounds called alkanes. Alkanes contain a single chain of carbon atoms with hydrogen atoms bonded along the side.

Hydrocarbons in crude oil • Three Principal Groups or Series of Hydrocarbon Compounds that Occur Naturally in Crude Oil. 1. paraffins 2. Aromatics 3. Naphthenes (cycloparaffins) 28

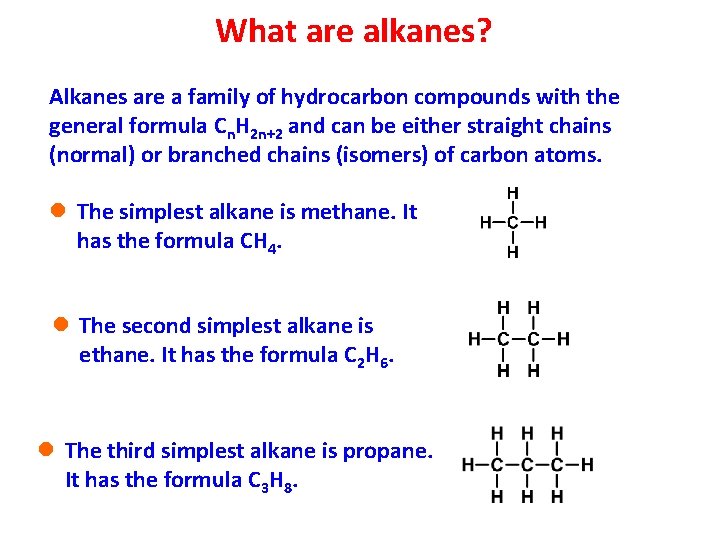
What are alkanes? Alkanes are a family of hydrocarbon compounds with the general formula Cn. H 2 n+2 and can be either straight chains (normal) or branched chains (isomers) of carbon atoms. l The simplest alkane is methane. It has the formula CH 4. l The second simplest alkane is ethane. It has the formula C 2 H 6. l The third simplest alkane is propane. It has the formula C 3 H 8.

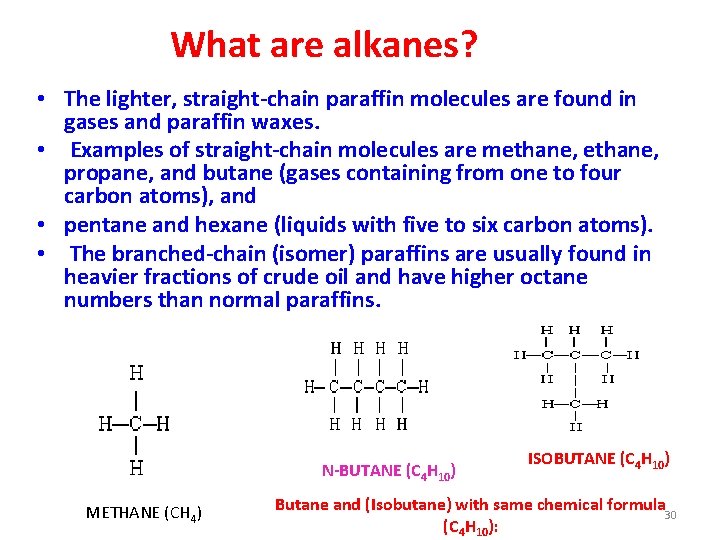
What are alkanes? • The lighter, straight-chain paraffin molecules are found in gases and paraffin waxes. • Examples of straight-chain molecules are methane, propane, and butane (gases containing from one to four carbon atoms), and • pentane and hexane (liquids with five to six carbon atoms). • The branched-chain (isomer) paraffins are usually found in heavier fractions of crude oil and have higher octane numbers than normal paraffins. N-BUTANE (C 4 H 10) METHANE (CH 4) ISOBUTANE (C 4 H 10) Butane and (Isobutane) with same chemical formula 30 (C 4 H 10):

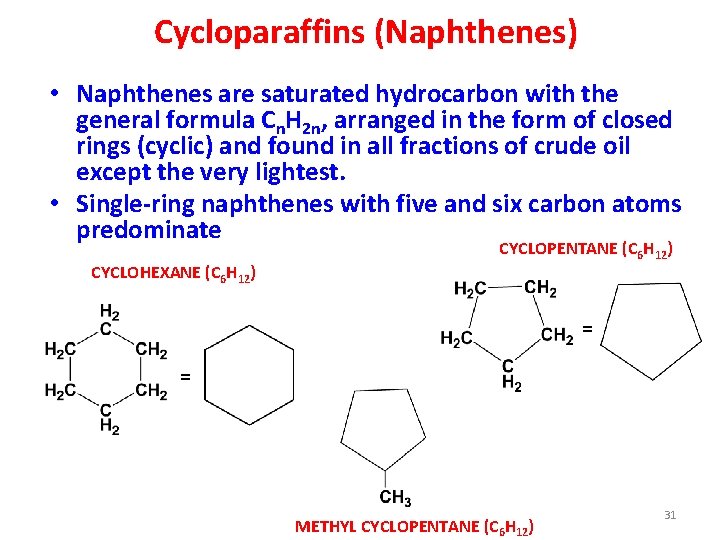
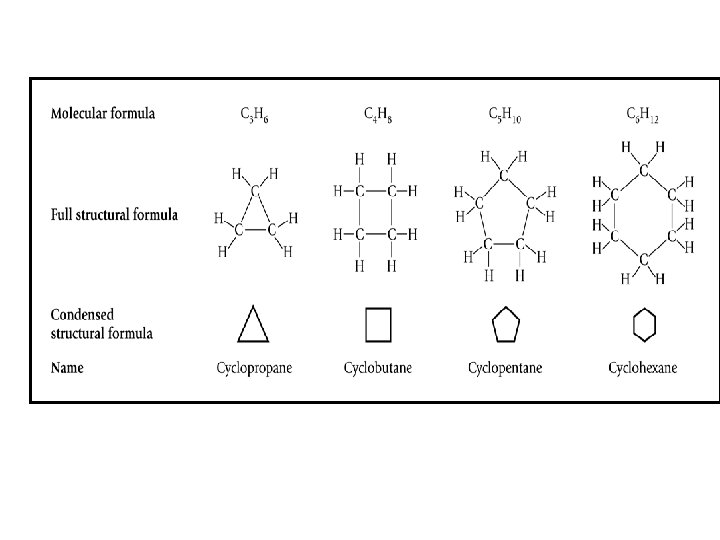
Cycloparaffins (Naphthenes) • Naphthenes are saturated hydrocarbon with the general formula Cn. H 2 n, arranged in the form of closed rings (cyclic) and found in all fractions of crude oil except the very lightest. • Single-ring naphthenes with five and six carbon atoms predominate CYCLOHEXANE (C 6 H 12) CYCLOPENTANE (C 6 H 12) = = METHYL CYCLOPENTANE (C 6 H 12) 31


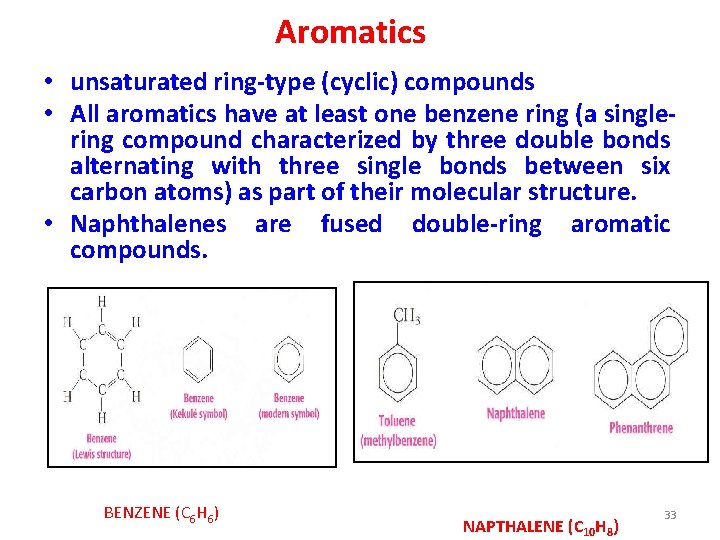
Aromatics • unsaturated ring-type (cyclic) compounds • All aromatics have at least one benzene ring (a singlering compound characterized by three double bonds alternating with three single bonds between six carbon atoms) as part of their molecular structure. • Naphthalenes are fused double-ring aromatic compounds. BENZENE (C 6 H 6) NAPTHALENE (C 10 H 8) 33

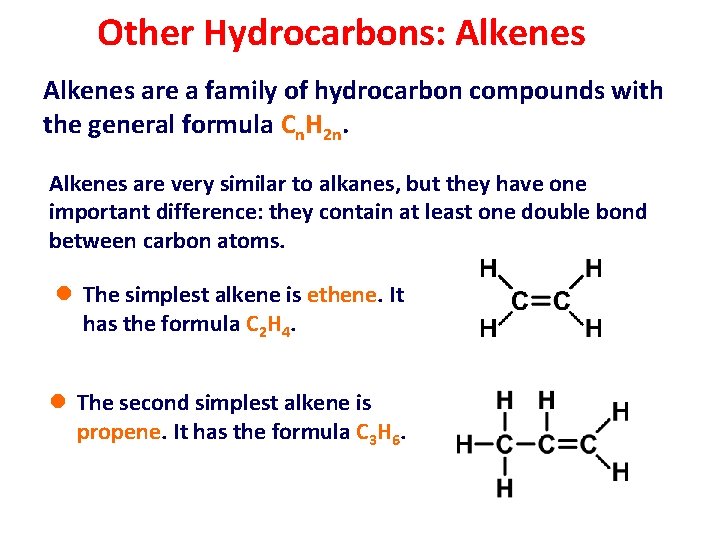
Other Hydrocarbons: Alkenes are a family of hydrocarbon compounds with the general formula Cn. H 2 n. Alkenes are very similar to alkanes, but they have one important difference: they contain at least one double bond between carbon atoms. l The simplest alkene is ethene. It has the formula C 2 H 4. l The second simplest alkene is propene. It has the formula C 3 H 6.

Non-hydrocarbons (a) Sulfur Compounds. Sulfur may be present in crude oil as hydrogen sulfide (H 2 S), as compounds (e. g. mercaptans, sulfides, disulfides, thiophenes, etc. ) or as elemental sulfur. • Each crude oil has different amounts and types of sulfur compounds, but as a rule the proportion, stability, and complexity of the compounds are greater in heavier crude-oil fractions. • Hydrogen sulfide is a primary contributor to corrosion in refinery processing units. Other corrosive substances are elemental sulfur and mercaptans. b. Oxygen Compounds • Oxygen compounds such as phenols, ketones, and carboxylic acids occur in crude oils in varying amounts. 35

Non-hydrocarbons c. Nitrogen Compounds. Nitrogen is found in lighter fractions of crude oil as basic compounds, and more often in heavier fractions of crude oil as nonbasic compounds that may also include trace metals such as copper, vanadium, and/or nickel. Nitrogen oxides can form in process furnaces. The decomposition of nitrogen compounds in catalytic cracking and hydrocracking processes forms ammonia and cyanides that can cause corrosion. 36

Non- hydrocarbons d. Trace Metals, including nickel, iron, and vanadium are often found in crude oils in small quantities and are removed during the refining process. Burning heavy fuel oils in refinery furnaces and boilers can leave deposits of vanadium oxide and nickel oxide in furnace boxes, ducts, and tubes. It is also desirable to remove trace amounts of arsenic, vanadium, and nickel prior to processing as they can poison certain catalysts. 37

Non- hydrocarbons e. Salts. Crude oils often contain inorganic salts such as sodium chloride, magnesium chloride, and calcium chloride in suspension or dissolved in water (brine). These salts must be removed or neutralized before processing to prevent catalyst poisoning, equipment corrosion.

THE END