EXTRACTION 1 Extraction is a unit operation in





















- Slides: 38

EXTRACTION 1

• Extraction, - is a unit operation in which separation of active constituents is achieved from a solid or liquid, preferably by a solvent (or menstrum) action. • Extraction may be defined as the treatment of plant or animal tissue with the solvent whereby medicinal active constituents are dissolved, the cell tissue and most of the inert matter remaining undissolved 2

• The solvent used for extraction is called menstruum • The undissolved residue left behind called marc. • The extracted preparation known as Galenicals. • Involves the separation of medicinally active portions of plant or animal tissues from the components (Material) by using selective solvents.

Types of extraction: General Methods of Extraction of drug can be done by following ways: 1. Infusion 2. Decoction 3. Maceration 4. Percolation 5. Digestion

• Infusion finally divided drug is treated with either hot or cold water for a certain length of time(15 -30 min), with occasional stirring after which the fluid portion is strained off and retained and the solid portion rejected. Apparatus used for this: coffee or tea pot (infusion pot) • Decoction active principle is extracted by boiling in water. For hard drugs & woody, thermostable

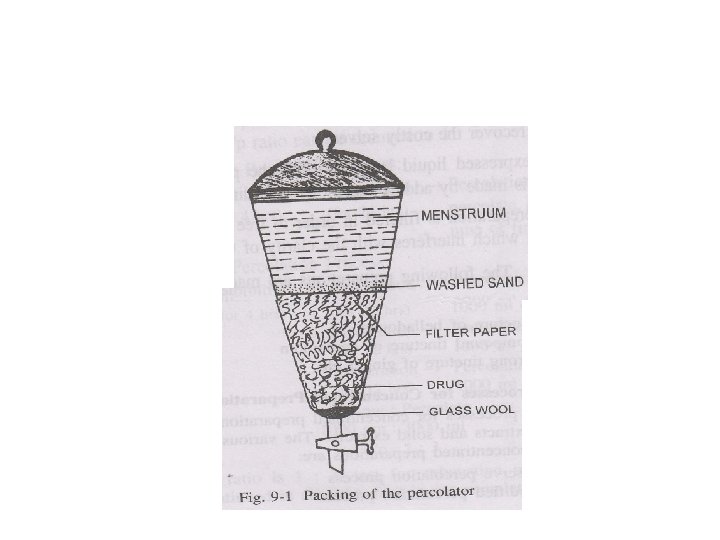
• Percolation In this process the drug is packed in a conical vessel (a percolator) with a small outlet at its lower end and moistened with the solvent which is added from time to time, and allowed to run off slowly from the lower outlet until a certain quantity of solvent has passed through. The marc is usually pressed out and the fluid obtained added to the percolate. • Expression In this process the drug is subjected to pressure and thus its juices are obtained.


Maceration • In this process the drug is placed with the whole of the menstrum in a closed vessel for 2 -7 days with occasional shaking. After 2 -7 days the liquid is removed & marc is pressed. Examples are: Tincture of orange, Tincture of squill. • In this process, the whole or coarsely powdered crude drug is placed in a closed container with the solvent and allowed to stand at room temperature for a period of at least 2 -7 days with frequent agitation until the soluble matter has dissolved. • The mixture then is strained, the marc (the damp solid material) is pressed, and the combined liquids are clarified by filtration Maceration process divided into two types: 1. Double maceration 2. Multiple maceration

• V) Digestion • drug is extracted by heating at a particular pressure • It is used when moderately elevated temperature is not objectionable. • The solvent efficiency of the menstrum is thereby increased.

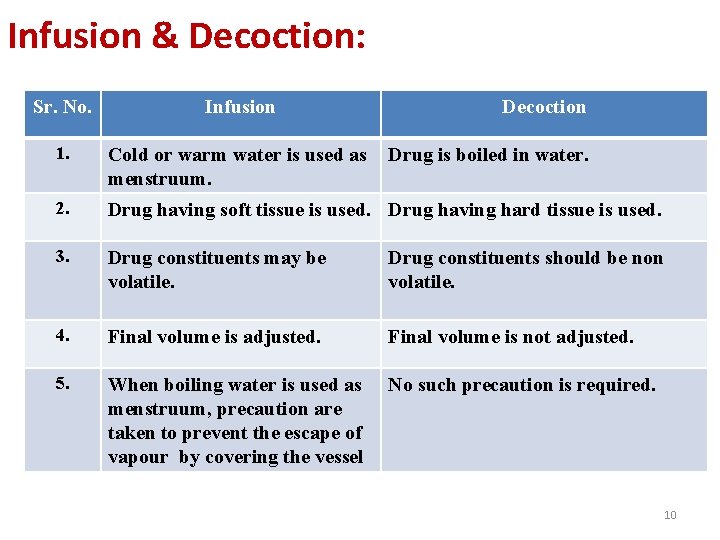
Infusion & Decoction: Sr. No. Infusion Decoction 1. Cold or warm water is used as menstruum. 2. Drug having soft tissue is used. Drug having hard tissue is used. 3. Drug constituents may be volatile. Drug constituents should be non volatile. 4. Final volume is adjusted. Final volume is not adjusted. 5. When boiling water is used as menstruum, precaution are taken to prevent the escape of vapour by covering the vessel No such precaution is required. Drug is boiled in water. 10

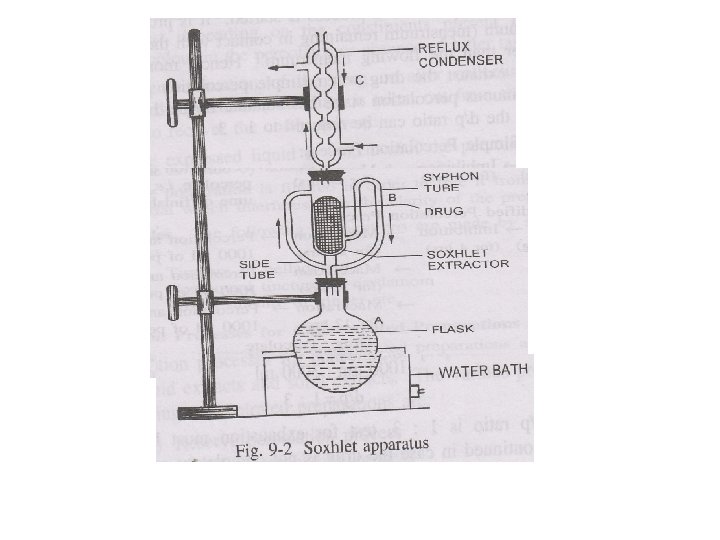
Continuous Hot Extraction (Soxhletion) • When active constituents of the drug are difficult to extract then it becomes necessary to extract the crude drug by hot menstrum for considerable period • E. g. , fixed oil from seed & alkaloids from drug are extracted by cont hot perco by organic solvent • Apparatus consists of three parts 1. flask containing boiling solvent 2. soxhlet extractor in which drug is packed 3. reflux condenser in which vapour of the solvent are condensed again into solvent


• • • Procedure: Drug is packed in soxhlet extractor Solvent is placed in flask then appa is fitted Boiled solvent converts into vapours, these vapours enters into condenser by through side tube and condensed into hot liquid which fall on the column of the drug Extractor filled with solvent, then level of syphon tube raises then solvent enters into flask Operation goes on continu until drug is exhausted LIMITATIONS 1. not suitable for drug which may block app. 2. only pure solvent 3. unsuitable for thermolabile dru

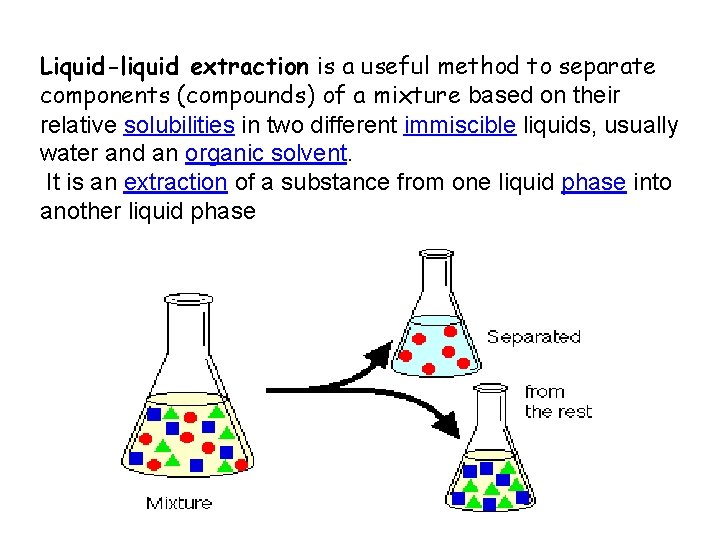
Liquid-liquid extraction is a useful method to separate components (compounds) of a mixture based on their relative solubilities in two different immiscible liquids, usually water and an organic solvent. It is an extraction of a substance from one liquid phase into another liquid phase

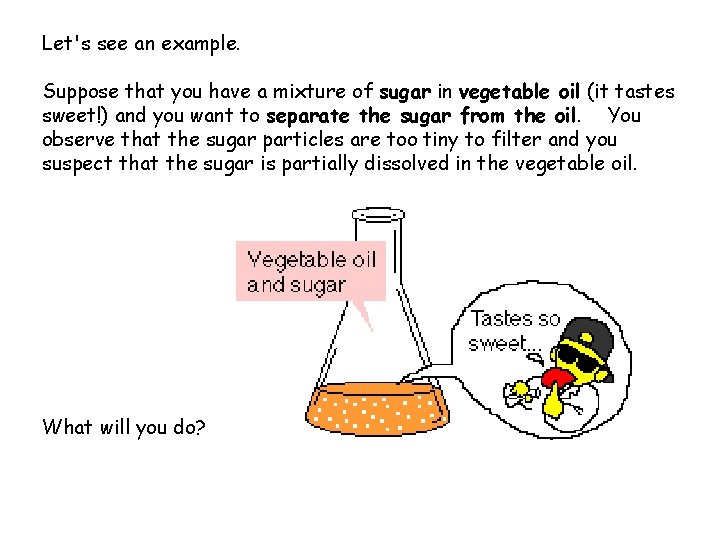
Let's see an example. Suppose that you have a mixture of sugar in vegetable oil (it tastes sweet!) and you want to separate the sugar from the oil. You observe that the sugar particles are too tiny to filter and you suspect that the sugar is partially dissolved in the vegetable oil. What will you do?

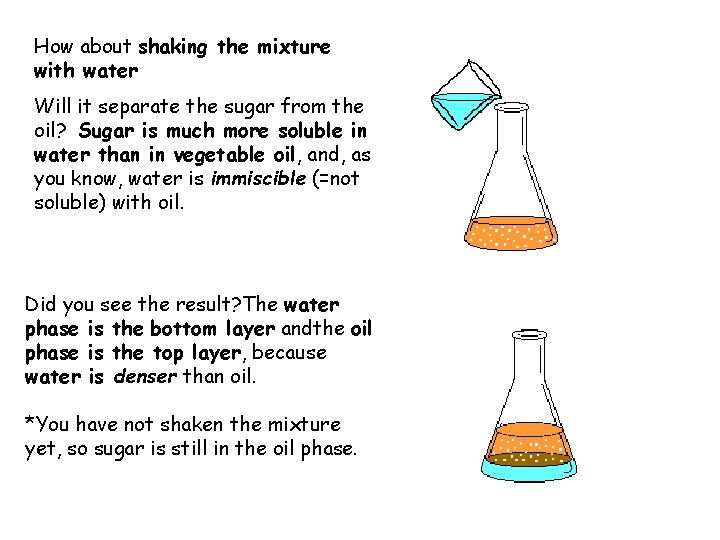
How about shaking the mixture with water Will it separate the sugar from the oil? Sugar is much more soluble in water than in vegetable oil, and, as you know, water is immiscible (=not soluble) with oil. Did you see the result? The water phase is the bottom layer andthe oil phase is the top layer, because water is denser than oil. *You have not shaken the mixture yet, so sugar is still in the oil phase.

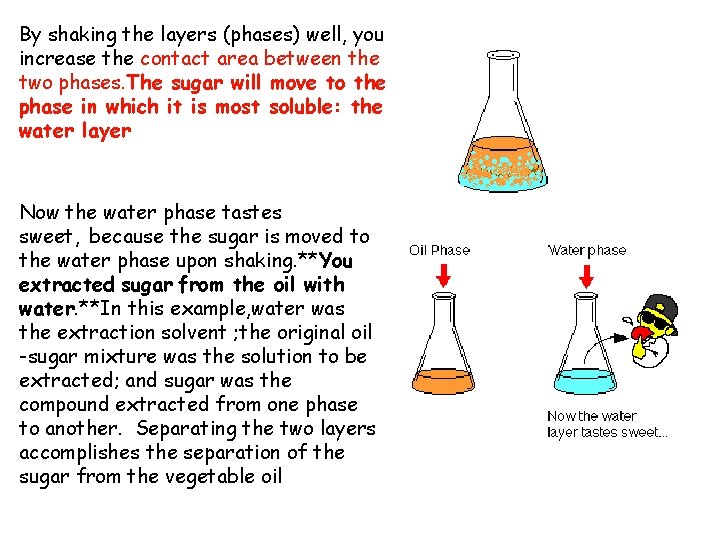
By shaking the layers (phases) well, you increase the contact area between the two phases. The sugar will move to the phase in which it is most soluble: the water layer Now the water phase tastes sweet, because the sugar is moved to the water phase upon shaking. **You extracted sugar from the oil with water. **In this example, water was the extraction solvent ; the original oil -sugar mixture was the solution to be extracted; and sugar was the compound extracted from one phase to another. Separating the two layers accomplishes the separation of the sugar from the vegetable oil

Liquid-liquid extraction is based on the transfer of a solute substance from one liquid phase into another liquid phase according to the solubility. Extraction becomes easy, by choosing a suitable solvent to separate a substance selectively from a mixture, or to remove unwanted impurities from a solution. In the practical use, usually one phase is a water or waterbased (aqueous) solution and the other an organic solvent which is immiscible with water. The success of this method depends upon the difference in solubility of a compound in various solvents.

Separatory Funnel Extraction Procedure Liquid–liquid extraction is a basic technique in chemical laboratories, where it is performed using a separatory funnel. Separatory funnels are designed to facilitate the mixing of immiscible liquids

• Factors affecting solid-liquid extraction Character of drug/nature of material: • Knowledge of p’cognosy is essential • Maceration is used, when drug is soft, unorganised/unpowderable • Percolation is used when drug is hard & tough Therapeutic value of drug • Max extraction is required when high TV • So percolation is used • Little TV---- maceration

• • • Particle size and distribution of the raw material: When the active principles are less readily extractable the drug is reduced to a powder before extraction but the correct particle size must be optimized. Due to shorter diffusionanl pathway smaller particle gives a faster rate of extraction. However too fine a particle size results in other difficulties Cost of drug: costly drugs are extracted by percolation and cheaply drug by maceration 21

Nature of solvent: following considerations are to be kept in mind while choosing a solvent. • From economic evaporation of the solvent from the extract the boiling pt should be minimum. • Chosen solvent should be non inflammable • In general, choice of solvent depends up on the physico chemical properties of the extractive and the need to reduce the loss of active principles. • It should be cheap. • For maceration water is used as a solvent • For percolation non-aq solvent • If active principles demand other solvent then gives the treatment 22

• • Temperature & Stability of Drugs Use of higher temperature during the extraction is avoided when the active constituents are thermolabile or volatile. if drugs are thermolabile cont hot percolation is avoided Maceration or percolation is the method Increased temperature hastens the process of diffusion and thus extraction. when solute remains in undissolved state then conti hot percolation/ hot solvent extraction Hot maceration accomplished by treating the drug in a closed vessel Soxhlet apparatus is used for the hot extraction Eg. , extraction of fixed oils from seed 23

Concentration of product: For dilute products such as tinctures is prepared by maceration and percolation For semi concentrated product, double or triple Maceration Liquid extract or dry extract -----percolation

Applications • Extraction is used in following area 1. Medicinal constituents are extracted from plant and animal tissue with organic solvent 2. Antibiotics are isolated from bacterial cultures by liquid-liquid extraction. 3. No of products like alkaloids, glycosides, proteins, fixed oils are produced by extraction.

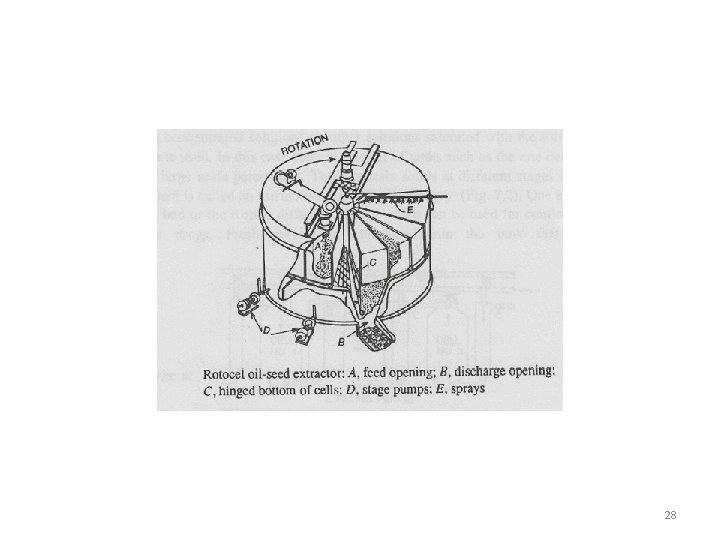
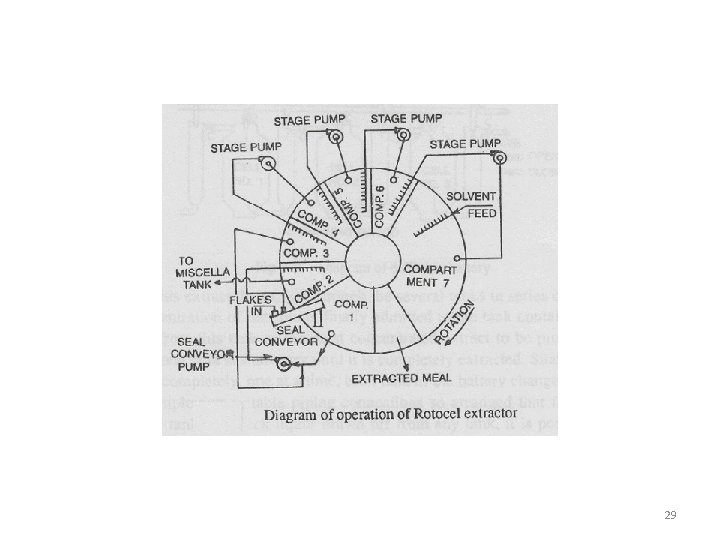
Solid – Liquid Extraction • Rotocel extractor • It is a continuous countercurrent percolator. • It consists of short cylindrical drum, enclosed in air tight housing. Rotated on a vertical axis • This cylinder is divided into several wedge-shaped compartments. Bottom of each compartment perforated and hinged • As the cylinder rotates on its vertical axis, a given compartment first comes under a opening where it is filled with the material to be extracted and then proceeds on to be treated with solvent in various stages of concentration. • After extraction the cell passes over an opening where the hinged bottom drops and discharges the exhausted material. 26

Rotocel Extractor 27

28

29

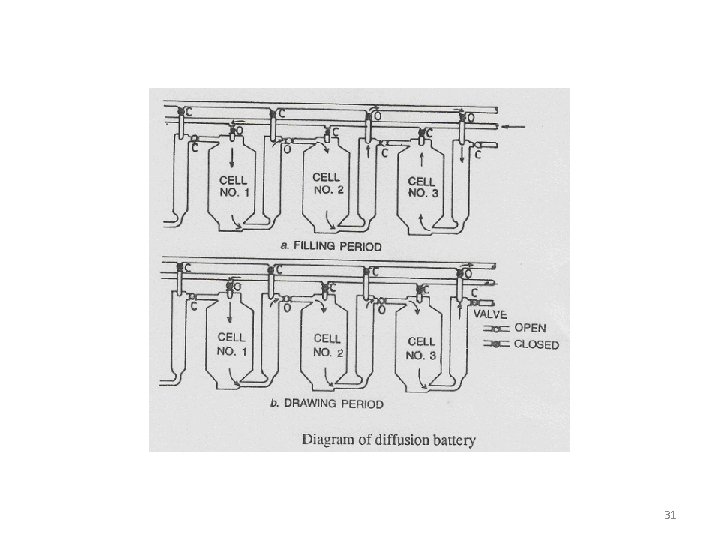
Diffusion Battery: • In this extractor there is a series of tanks which contain solids at different stages of extraction. • This is used for continuous extraction of vegetable drugs. • Fresh solvent is introduced into the tank that is most nearly exhausted and this extract then flows through the several tanks in series containing gradually increasing concentration of the solute. • Lastly admitted to the tank containing the fresh solid and drawn off from this tank as the most concentrated extract to be processed further 30

31

• Liquid- liquid extraction: • Extensively used in the isolation of antibiotics such as penicillin- G from aqueous fermentation broths. • It is also used in recovery of other fermentation products such as lactic acid and synthetic drugs. • In this extraction two liquids are used. • The heavy phase, usually the aqueous, is introduced at the top and flows downward while the lighter phase normally the organic solvent is introduced at the bottom and flows upward. • Various methods are used to bring the liquid phases into intimate contact with each other. 32

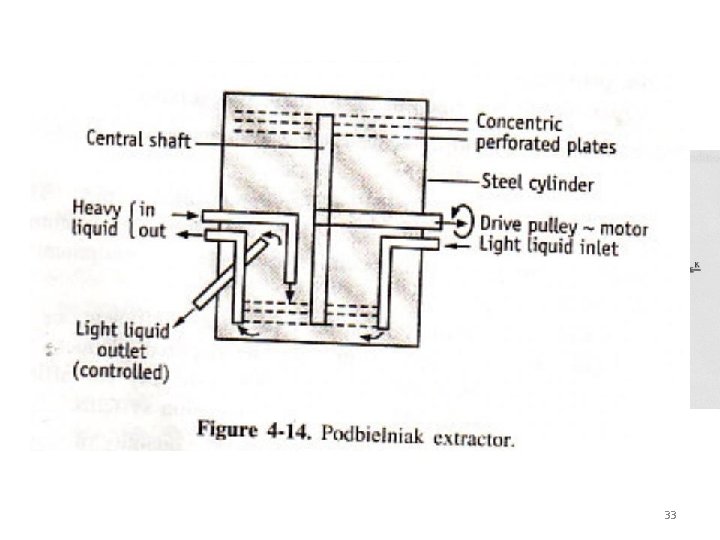
• Podbielniak extractor: • • • • It consists of – ‘A’ steel cylinder ‘B’ concentric rings of perforated plates ‘C’ inlet for heavier liquid ‘D’ Ports for heavier liquid ‘E’ collecting space for heavier liquid ‘F’ outlet channels for heavier liquid ‘G’ inlet for lighter liquid ‘H’ collecting space for lighter liquid ‘I’ outlet for lighter liquid ‘J’ exit for light liquid ‘K’ inlet for was water ‘L’ stationary housing ‘M’ cleanout plugs. 33

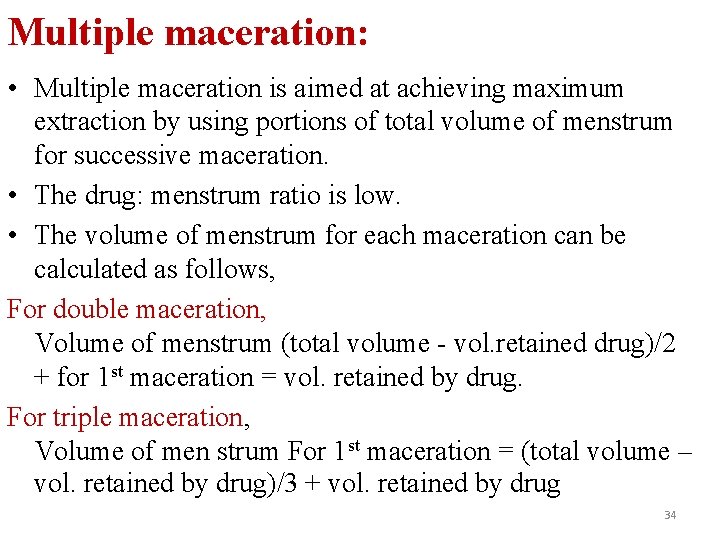
Multiple maceration: • Multiple maceration is aimed at achieving maximum extraction by using portions of total volume of menstrum for successive maceration. • The drug: menstrum ratio is low. • The volume of menstrum for each maceration can be calculated as follows, For double maceration, Volume of menstrum (total volume - vol. retained drug)/2 + for 1 st maceration = vol. retained by drug. For triple maceration, Volume of men strum For 1 st maceration = (total volume – vol. retained by drug)/3 + vol. retained by drug 34

• Hot Continuous Extraction (Soxhletion) • In this method, the finely ground crude drug is placed in a porous bag or “thimble” made of strong filter paper, which is placed in chamber E of the Soxhlet apparatus. • The extracting solvent in flask A is heated and its vapors condense in condenser D. The condensed extractant drips into the thimble containing the crude drug, and extracts it by contact. • When the level of liquid in chamber E rises to the top of siphon tube C, the liquid contents of chamber E siphon into fl ask A. • This process is continuous and is carried out until a drop of solvent from the siphon tube does not leave residue when evaporated. • The advantage of this method, compared to previously described methods, is that large amounts of drug can be extracted with a much smaller quantity of solvent. • This effects tremendous economy in terms of time, energy and consequently financial inputs. • At small scale, it is employed as a batch process only, but it becomes much more economical and viable when converted into a continuous extraction procedure on medium or large scale.

Modified maceration: • It is essentially used for extracting unorganized drugs. Ex: gums, resins etc. • This process is quick because the soluble constituents are directly exposed to menstruum due to lack of cellular structure. • The final product is adjusted to definite volume. • The filtrate should be collected in a dry receiver or in a receiver rinsed with menstrum because the resinous matter present in most of the unorganized drugs is insoluble in water affecting the clarity of the final product. Ex: preparation of tincture of opium.

Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds based on their relative solubilities in two different immiscible liquids, usually water and an organic solvent.

b) Mechanism and method of leaching: • • • Several resistances are encountered when the process of extraction is carried out such as. Menstrum should diffuse from main suspension to the surface of the cell. Diffusion of menstrum through the cell wall to the site of the active constituent. Dissolution of the active constituent. Diffusion of the solution through the cell wall to the outside. 38