EXTRACTING METALS FROM THEIR ORES CONTENTS What Are















- Slides: 17

EXTRACTING METALS FROM THEIR ORES

CONTENTS • • • What Are Ores ? Metal Ores How To Extract Metals From Ores ? Reduction Of Metal Ores Effects On Environment

WHAT ARE ORES ? • An ore is any naturallyoccurring source of a metal that you can economically extract the metal from. • Aluminium, for example, is the most common metal in the Earth's crust, occurring in all sorts of minerals. An Ore

METAL ORES • Metals are usually found in ores containing mineral. • They are combined with Oxygen or Sulphur to form Oxides or Sulphides. • Sulphide Ores are converted to Oxides by heating them in air (called roasting) • Eg : Zinc Sulphide to Zinc Oxide Zinc Sulphide Oxygen Zinc Oxide Zn. S (s) 1 ½ O 2 (g) Zn. O (s) Sulphur Dioxide SO 2 (g)

HOW TO EXTRACT METALS FROM ORES ? • To obtain a metal , it’s ore , a reducing agent is used to remove the Oxygen , in a reduction reaction. • Examples of some reducing agents : An impure form of carbon Made from Methane and Water

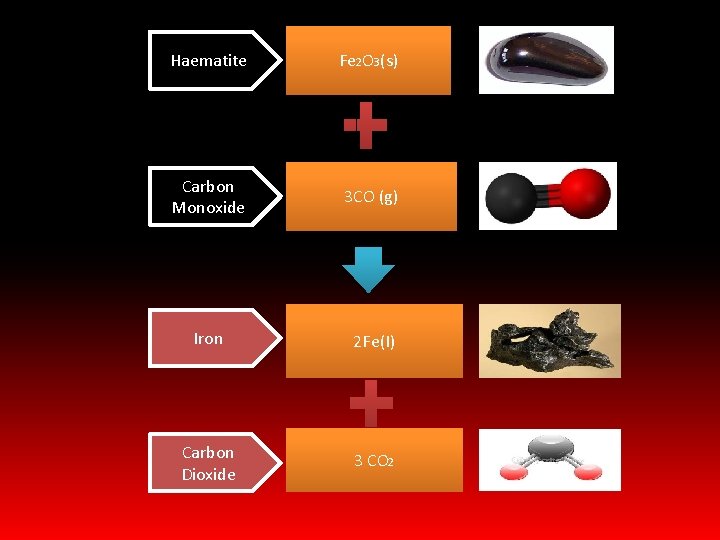
REDUCTION OF METAL ORES • Iron , Manganese And Copper Ø Carbon and Carbon monoxide are the reducing agents in the extraction of Fe , Mn & Cu Ø These are cheap because coke can be used which is cheaply produced by heating coal in absence of air Ø Extraction of Iron is done in a blast furnace which reaches temperatures of 2000 K

Haematite Fe 2 O 3(s) Carbon Monoxide 3 CO (g) Iron 2 Fe(I) Carbon Dioxide 3 CO 2

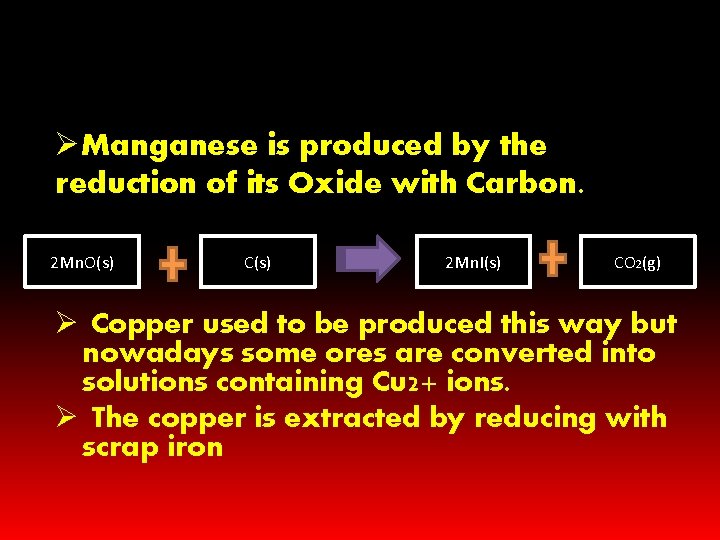
ØManganese is produced by the reduction of its Oxide with Carbon. 2 Mn. O(s) C(s) 2 Mn. I(s) CO 2(g) Ø Copper used to be produced this way but nowadays some ores are converted into solutions containing Cu 2+ ions. Ø The copper is extracted by reducing with scrap iron

Ø This is “greener” as no CO 2 is produced and scrap iron is cheap and readily available Ø Also , solution can be made from low grade ore so it is readily available

• Extracting Of Other Metals Ø Some metals can’t be extracted using carbon reduction such as Aluminium , Titanium , and Tungsten. Ø Aluminium cannot be extracted because it is more reactive than carbon. Ø Titanium and Tungsten also can’t be extracted using carbon because they will form a metal carbide which would make the metal brittle.

• Extraction Of Aluminium Ø Aluminium is extracted from purified bauxite ore (mainly Al 2 O 3) Ø Al 2 O 3 is dissolved in molten cryolite , which forms a solution which melts at around 1240 K (Al 2 O 3 melts at 2435 K) Ø Therefore less energy is required

Ø Aluminium is produced at the negative electrode Ø Oxygen is produced at the positive electrode Ø This gives us an equation : Al 2 O 3(I) 2 Al(I) 1 ½ O(g)

• Extraction Of Titanium Ø As Titanium can’t be reduced by Carbon it is reduced by Sodium or Magnesium. Ø It is an expensive process as Titanium Oxide is first converted to Titanium Chloride by reacting it with coke and Chlorine at 1173 K

Ø Titanium Chloride is then reduced by Sodium or Magnesium into Titanium under an inert argon atmosphere at 1300 K

• Extraction Of Tungsten Ø Tungsten is extracted from it’s Oxide , WO 3 by reduction with H 2 at high temp. Ø However , there is some risk as H 2 is a flammable gas , so using it as a reducing agent is a last resort

EFFECTS ON ENVIRONMENT • Recycling scrap metals has many environmental and economic advantage. • It reduces the amount of scrap metal in landfill and it has already been extracted from its ore so energy is not required again.

THANK YOU