Extracting Metals by Electrolysis 1 of 22 Boardworks
Extracting Metals by Electrolysis 1 of 22 © Boardworks Ltd 2011
2 of 22 © Boardworks Ltd 2011

Extracting metals from ores The method of extraction which is most appropriate depends on the reactivity of the metal being extracted. This can be discovered using the reactivity series. Metals above carbon in the reactivity series must be extracted using electrolysis. 3 of 22 increasing reactivity Most metals that we use are found combined with other elements, as compounds in ores. These metals must be extracted from their ores before they can be made useful. potassium sodium calcium magnesium aluminium (carbon) zinc iron lead (hydrogen) copper silver gold platinum © Boardworks Ltd 2011
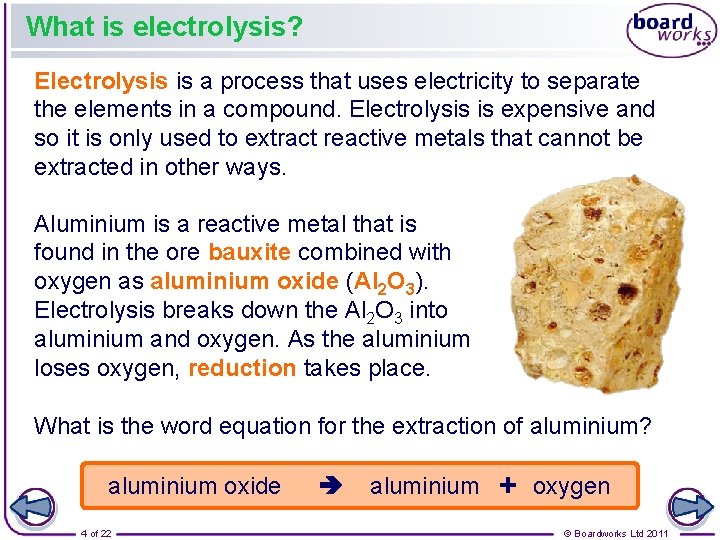
What is electrolysis? Electrolysis is a process that uses electricity to separate the elements in a compound. Electrolysis is expensive and so it is only used to extract reactive metals that cannot be extracted in other ways. Aluminium is a reactive metal that is found in the ore bauxite combined with oxygen as aluminium oxide (Al 2 O 3). Electrolysis breaks down the Al 2 O 3 into aluminium and oxygen. As the aluminium loses oxygen, reduction takes place. What is the word equation for the extraction of aluminium? aluminium oxide 4 of 22 aluminium + oxygen © Boardworks Ltd 2011
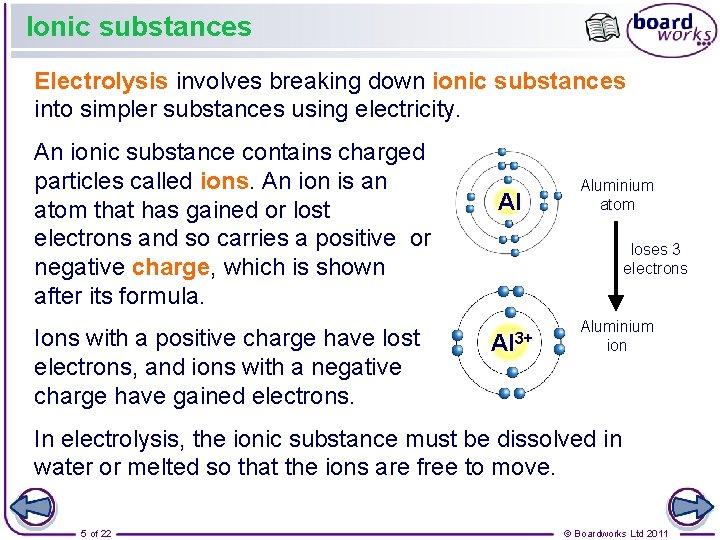
Ionic substances Electrolysis involves breaking down ionic substances into simpler substances using electricity. An ionic substance contains charged particles called ions. An ion is an atom that has gained or lost electrons and so carries a positive or negative charge, which is shown after its formula. Ions with a positive charge have lost electrons, and ions with a negative charge have gained electrons. Al Aluminium atom loses 3 electrons Al 3+ Aluminium ion In electrolysis, the ionic substance must be dissolved in water or melted so that the ions are free to move. 5 of 22 © Boardworks Ltd 2011
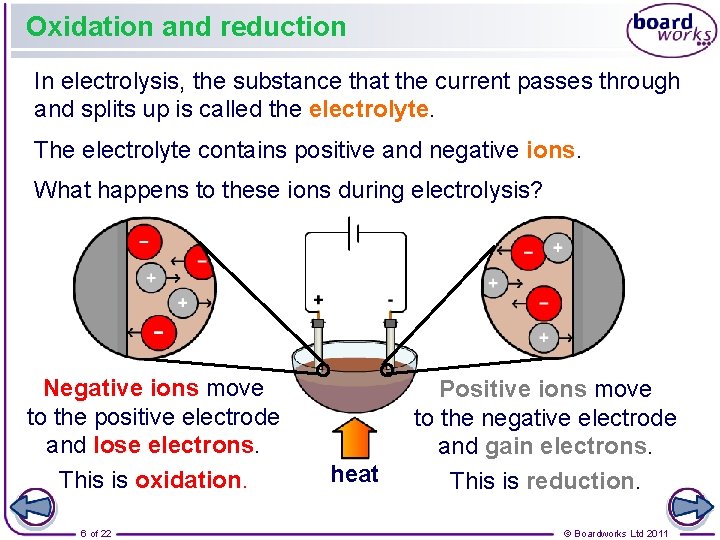
Oxidation and reduction In electrolysis, the substance that the current passes through and splits up is called the electrolyte. The electrolyte contains positive and negative ions. What happens to these ions during electrolysis? Negative ions move to the positive electrode and lose electrons. This is oxidation. 6 of 22 heat Positive ions move to the negative electrode and gain electrons. This is reduction. © Boardworks Ltd 2011
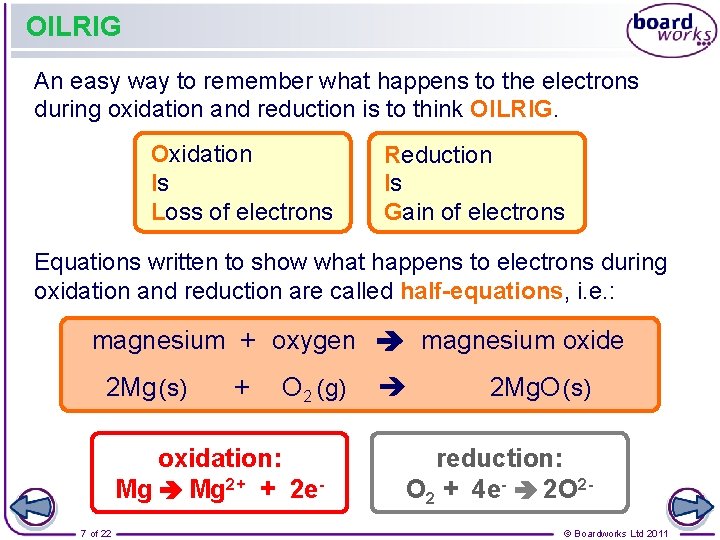
OILRIG An easy way to remember what happens to the electrons during oxidation and reduction is to think OILRIG. Oxidation Is Loss of electrons Reduction Is Gain of electrons Equations written to show what happens to electrons during oxidation and reduction are called half-equations, i. e. : magnesium + oxygen magnesium oxide 2 Mg (s) + O 2 (g) oxidation: Mg 2+ + 2 e 7 of 22 2 Mg. O (s) reduction: O 2 + 4 e- 2 O 2© Boardworks Ltd 2011

Extracting aluminium Aluminium is one of the most useful metals in the world. Electrolysis is used to extract aluminium from its ore. Why is it not possible to extract aluminium by heating its ore with carbon? Aluminium ore (bauxite) is more reactive than carbon and has a very high melting point (2050 °C). In electrolysis, the ore is dissolved in a compound called cryolite (Na 3 Al. F 6), which effectively lowers the melting point to 1, 000 °C. 8 of 22 © Boardworks Ltd 2011
Aluminium from bauxite 9 of 22 © Boardworks Ltd 2011
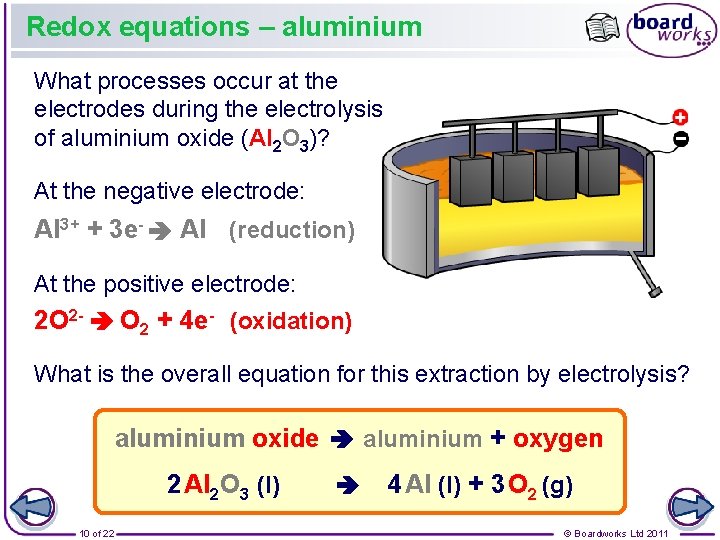
Redox equations – aluminium What processes occur at the electrodes during the electrolysis of aluminium oxide (Al 2 O 3)? At the negative electrode: Al 3+ + 3 e- Al (reduction) At the positive electrode: 2 O 2 - O 2 + 4 e- (oxidation) What is the overall equation for this extraction by electrolysis? aluminium oxide aluminium + oxygen 2 Al 2 O 3 (l) 10 of 22 4 Al (l) + 3 O 2 (g) © Boardworks Ltd 2011
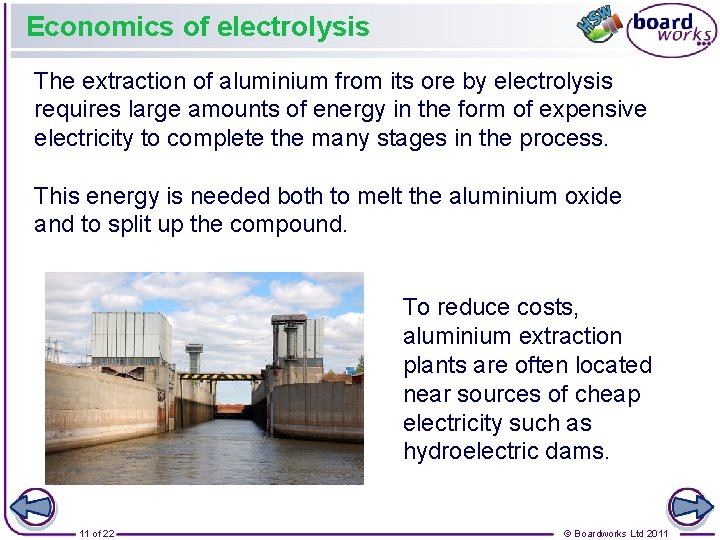
Economics of electrolysis The extraction of aluminium from its ore by electrolysis requires large amounts of energy in the form of expensive electricity to complete the many stages in the process. This energy is needed both to melt the aluminium oxide and to split up the compound. To reduce costs, aluminium extraction plants are often located near sources of cheap electricity such as hydroelectric dams. 11 of 22 © Boardworks Ltd 2011
Extracting aluminium summary 12 of 22 © Boardworks Ltd 2011
13 of 22 © Boardworks Ltd 2011
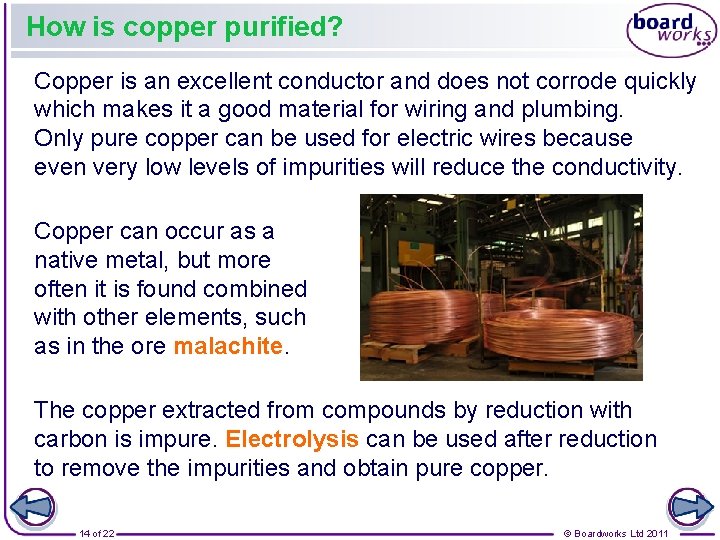
How is copper purified? Copper is an excellent conductor and does not corrode quickly which makes it a good material for wiring and plumbing. Only pure copper can be used for electric wires because even very low levels of impurities will reduce the conductivity. Copper can occur as a native metal, but more often it is found combined with other elements, such as in the ore malachite. The copper extracted from compounds by reduction with carbon is impure. Electrolysis can be used after reduction to remove the impurities and obtain pure copper. 14 of 22 © Boardworks Ltd 2011
Purifying copper using electrolysis 15 of 22 © Boardworks Ltd 2011
Labelling copper purification 16 of 22 © Boardworks Ltd 2011
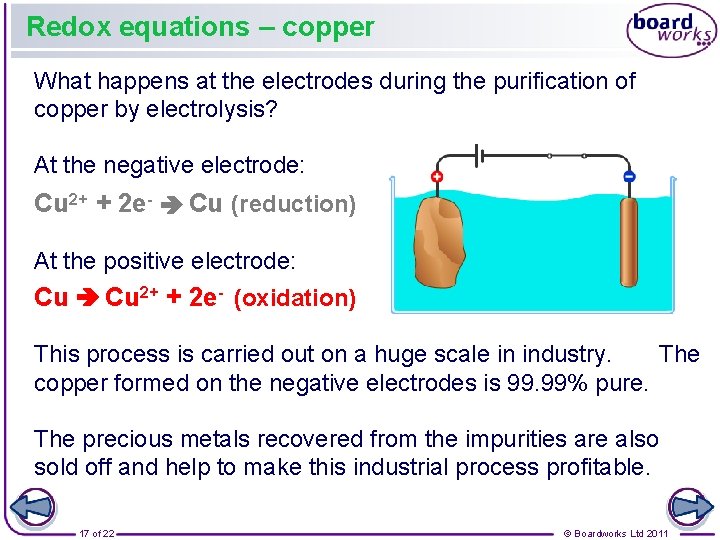
Redox equations – copper What happens at the electrodes during the purification of copper by electrolysis? At the negative electrode: Cu 2+ + 2 e- Cu (reduction) At the positive electrode: Cu 2+ + 2 e- (oxidation) This process is carried out on a huge scale in industry. The copper formed on the negative electrodes is 99. 99% pure. The precious metals recovered from the impurities are also sold off and help to make this industrial process profitable. 17 of 22 © Boardworks Ltd 2011
Purifying copper – true or false? 18 of 22 © Boardworks Ltd 2011
19 of 22 © Boardworks Ltd 2011
Glossary 20 of 22 © Boardworks Ltd 2011
Anagrams 21 of 22 © Boardworks Ltd 2011
Multiple-choice quiz 22 of 22 © Boardworks Ltd 2011
- Slides: 22