EXTRACELLULAR MATRIX Extracellular matrix ECM cells mesenchymal origin

![Collagen Distribution Type Molecule composition Tissue Fibrilar Collagens I [a 1(I)2 a 2(I)] Skin, Collagen Distribution Type Molecule composition Tissue Fibrilar Collagens I [a 1(I)2 a 2(I)] Skin,](https://slidetodoc.com/presentation_image_h/f93ea43fb990c25b51fd3104e551d899/image-15.jpg)


- Slides: 25

EXTRACELLULAR MATRIX

Extracellular matrix (ECM) Ø cells (mesenchymal origin) - fibroblasts - smooth muscle cells - chondroblasts - osteoblasts and epitelial cells Ø organic fibrilar matrix Ø organic nonfibrilar matrix Ø water

Function Ø Support for cells regulates and determine: - polarity - cell differentiation - adhesion - migration Ø Mechanical support for tissues and organ architecture - growth - regenerative and healing processes - determination and maintenance of the structure Ø Exchange of different metabolites, ions, water

Structure Ø collagen – the main ECM component, main fibres Ø elastin Ø proteoglycans - heteropolysacharides Ø structural glycoproteins - fibronectin, laminin

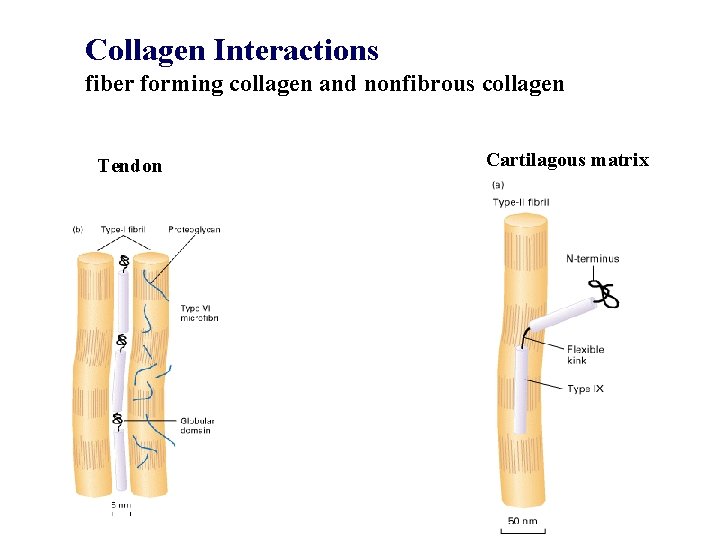
Collagen The architectural structure of collagen determines the function of collagen fibers as mechanical reinforcements of connective tissues (tendon, skin, bone, arteries etc. ). Tendon fibers are bundles of uniaxially aligned fibers. Skin (dermis) is a random planar array of collagen fibers. Bone is a hydroxyapatite which is reinforced by collagen fibers. Large blood vessels (aorta, large arteries) are interpenetrating networks of elastin fibers and collagen fibers.

Structure Ø Insoluble glycoprotein - protein + carbohydrate 30% of all body proteins (by weight) - G – X – A – G – Y – A – G – A – G – X – A – G – Y – A – G – A – A – G – X – A – G – Y – A – G – X – A – G - glycine, X - proline nebo hydroxyproline, Y – lysin nebo hydroxylysin, A – amino acid Ø hydroxyproline (25% of total AA) stifness polypeptide chain Ø carbohydrate - glucose - galactose

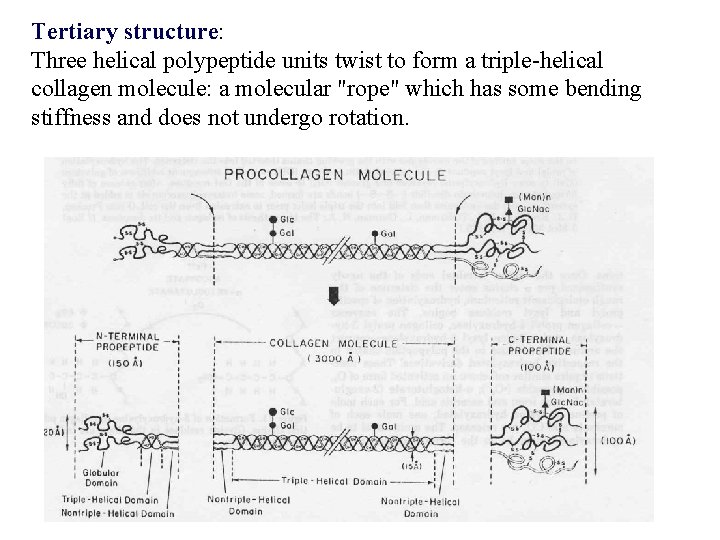
Tertiary structure: Three helical polypeptide units twist to form a triple-helical collagen molecule: a molecular "rope" which has some bending stiffness and does not undergo rotation.

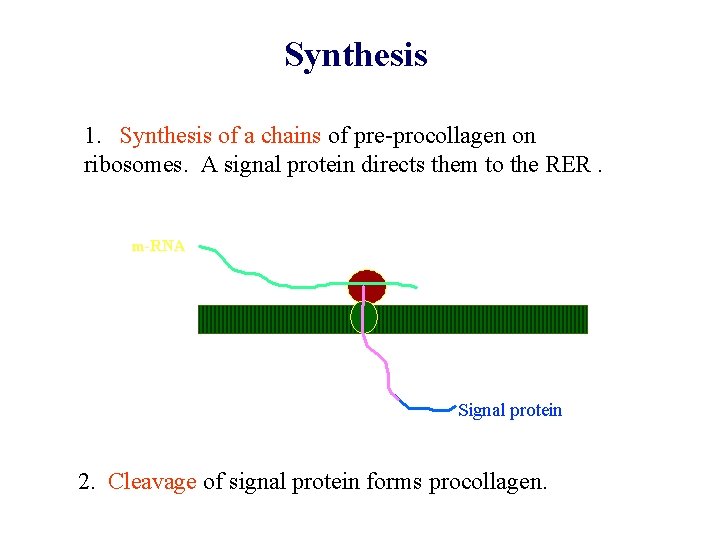
Synthesis 1. Synthesis of a chains of pre-procollagen on ribosomes. A signal protein directs them to the RER. 2. Cleavage of signal protein forms procollagen.

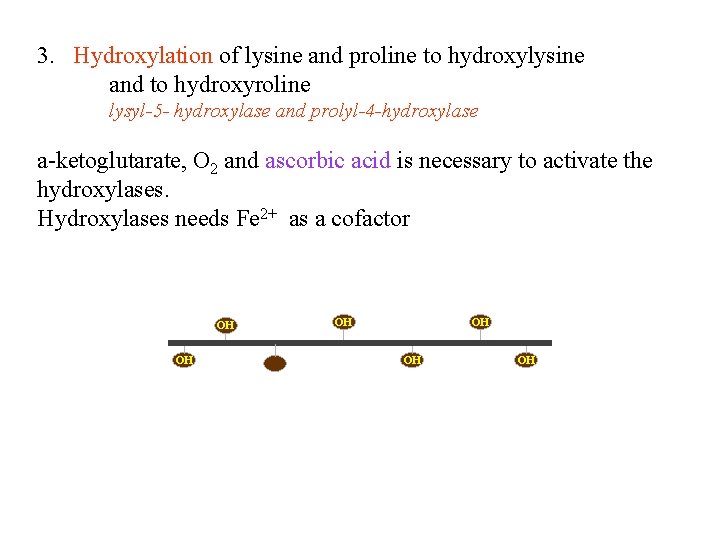
3. Hydroxylation of lysine and proline to hydroxylysine and to hydroxyroline lysyl-5 - hydroxylase and prolyl-4 -hydroxylase a-ketoglutarate, O 2 and ascorbic acid is necessary to activate the hydroxylases. Hydroxylases needs Fe 2+ as a cofactor

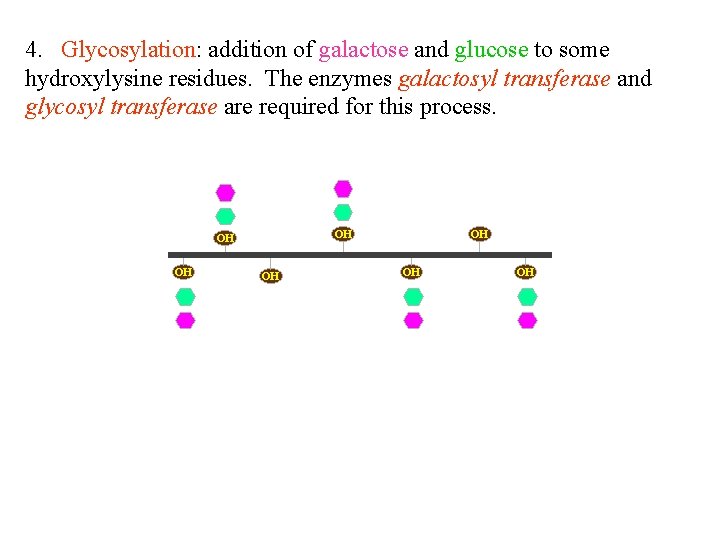
4. Glycosylation: addition of galactose and glucose to some hydroxylysine residues. The enzymes galactosyl transferase and glycosyl transferase are required for this process.

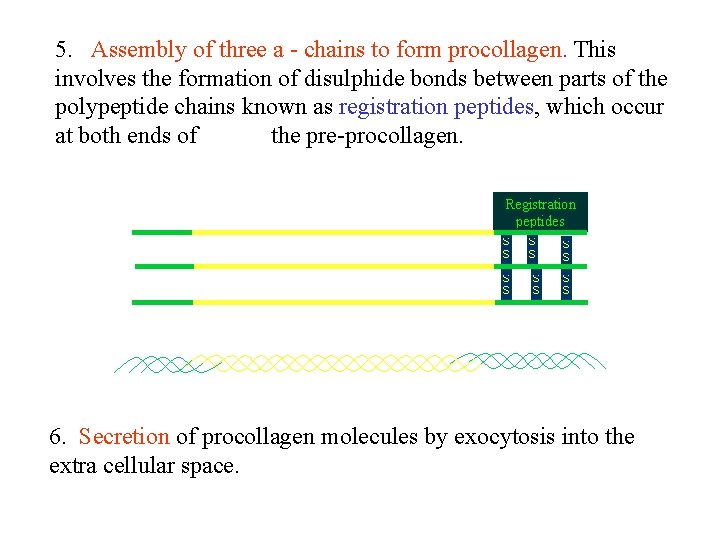
5. Assembly of three a - chains to form procollagen. This involves the formation of disulphide bonds between parts of the polypeptide chains known as registration peptides, which occur at both ends of the pre-procollagen. 6. Secretion of procollagen molecules by exocytosis into the extra cellular space.

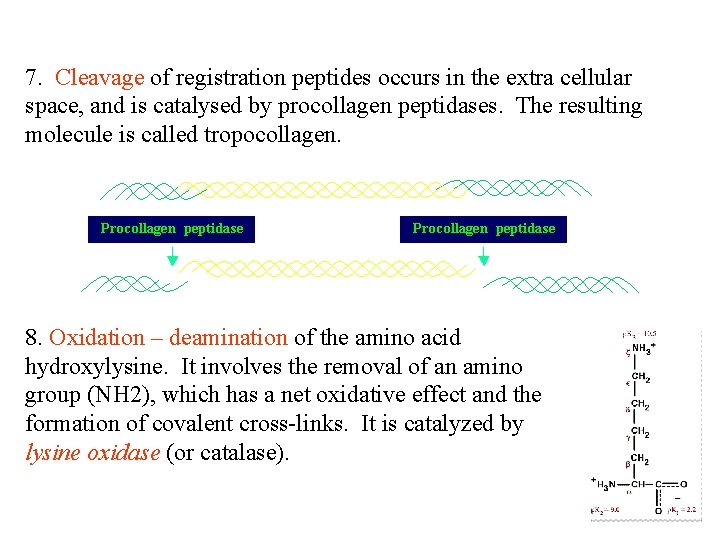
7. Cleavage of registration peptides occurs in the extra cellular space, and is catalysed by procollagen peptidases. The resulting molecule is called tropocollagen. 8. Oxidation – deamination of the amino acid hydroxylysine. It involves the removal of an amino group (NH 2), which has a net oxidative effect and the formation of covalent cross-links. It is catalyzed by lysine oxidase (or catalase).

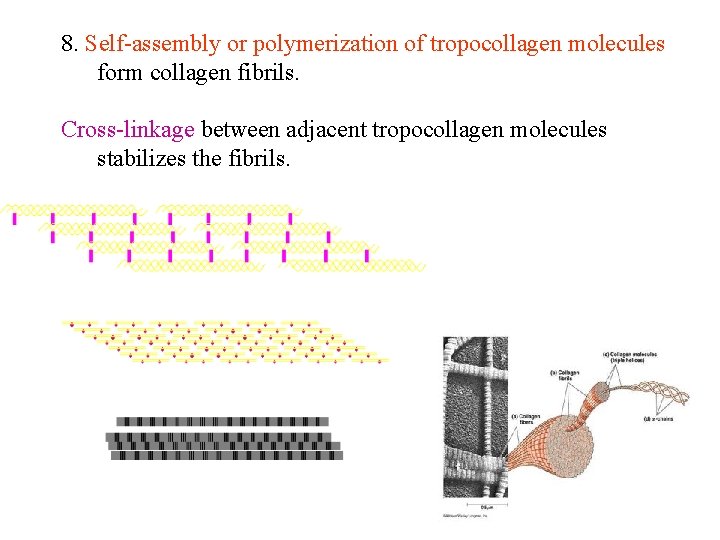
8. Self-assembly or polymerization of tropocollagen molecules form collagen fibrils. Cross-linkage between adjacent tropocollagen molecules stabilizes the fibrils.

Collagen Interactions fiber forming collagen and nonfibrous collagen Tendon Cartilagous matrix
![Collagen Distribution Type Molecule composition Tissue Fibrilar Collagens I a 1I2 a 2I Skin Collagen Distribution Type Molecule composition Tissue Fibrilar Collagens I [a 1(I)2 a 2(I)] Skin,](https://slidetodoc.com/presentation_image_h/f93ea43fb990c25b51fd3104e551d899/image-15.jpg)
Collagen Distribution Type Molecule composition Tissue Fibrilar Collagens I [a 1(I)2 a 2(I)] Skin, tendon, bone, ligaments, dentin II [a 1(II)]3 Cartilage, vitreus humor III [a 1(III)]3 Skin, muscle, blood vessels V [a 1(V)]3 Similar to type I, fetal tissue Sheet-forming collagen IV [a 1(IV)2 a 2(IV)] [a 1(IV)a 2(IV)a 3(IV)] All basal laminaes

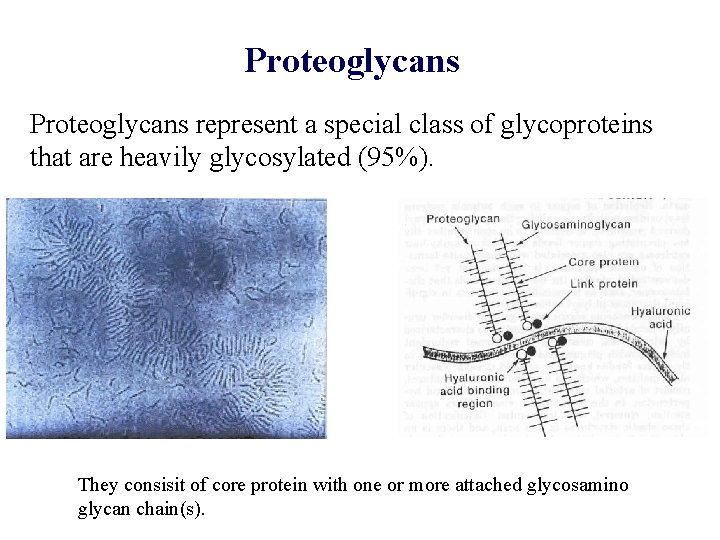
Proteoglycans represent a special class of glycoproteins that are heavily glycosylated (95%). They consisit of core protein with one or more attached glycosamino glycan chain(s).

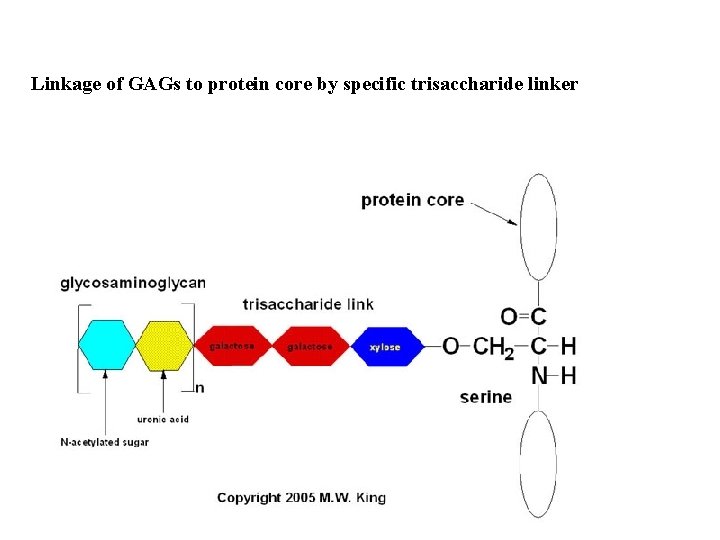
Glycosaminoglycans (GAG) Glycosaminoglycan (GAG) chains are long, linear carbohydrate polymers under physiological conditions they are negatively charged (due to the occurrence of sulfate and uronic acid groups). Disaccharide subunits are: - uronic acid D-glucuronic acid or L-iduronic acid - aminosugar N-acetyl glucosamin (Glc. NAc) or N-acetyl galactosamin (Gal. NAc)

Linkage of GAGs to protein core by specific trisaccharide linker

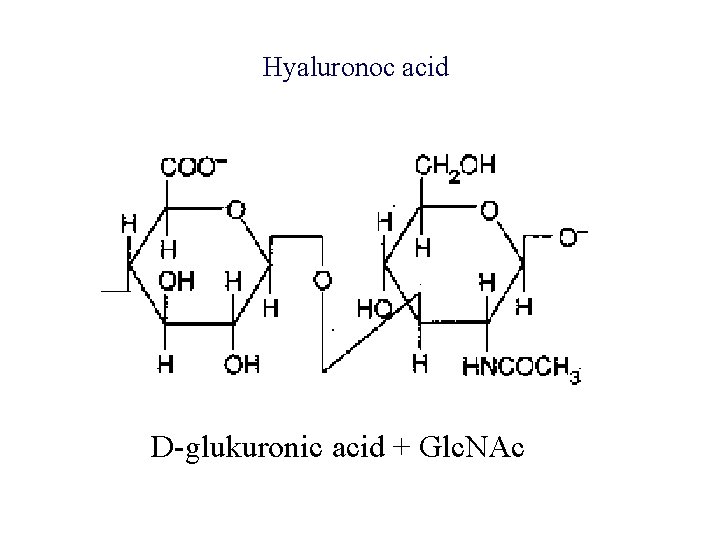
Hyaluronoc acid D-glukuronic acid + Glc. NAc

Glycosaminoglycan classification Proteoglycans can be categorised depending upon the nature of their glycosaminoglycan chains. Ø Hyaluronic acid (does not contain any sulfate) - non-covalent link complex with proteoglycans Ø Chondroitin sulfate cartilage, bone Ø Dermatan sulfate skin, blood vessels Ø Heparan sulfate basement membrane, component of cells surface Ø Keratan sulfate cornea, bone, cartilage - often aggregated with chondroitin sulfate

Function • organize water molecules - resistens to compresion - return to original shape - repel negative molecules • occupy space between cells and collagen • high viscosity - lubricating fluid inthe joints • specific binding to other macromolecules • link to collagen fibres - form network - in bone combine with calcium salts (calcium carbonate, hydroxyapatite) • cell migration and adhesion - passageways between cells • anchore cells to matrix fibers

Structural Glycoproteins Ø Direct linkage to collagen or proteoglycans - insertion of fibers to membrane - covalent attachment to membrane lipid Ø Linking glycoproteins -fibronectin - laminin

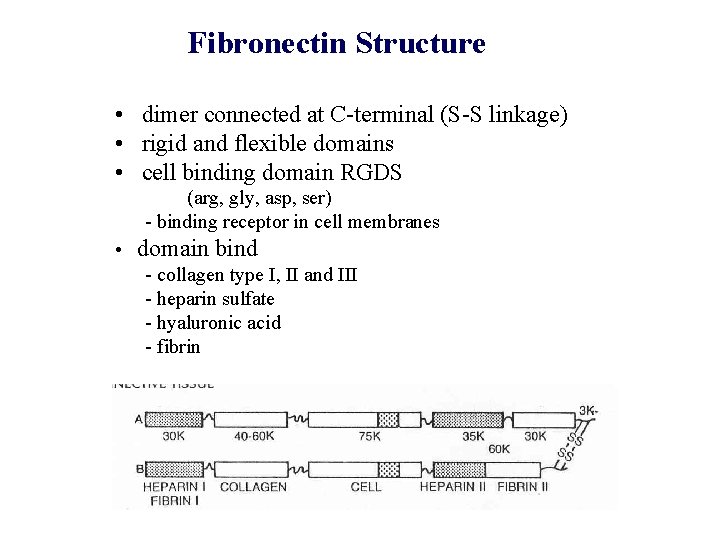
Fibronectin Structure • dimer connected at C-terminal (S-S linkage) • rigid and flexible domains • cell binding domain RGDS (arg, gly, asp, ser) - binding receptor in cell membranes • domain bind - collagen type I, II and III - heparin sulfate - hyaluronic acid - fibrin

Fibronectin Function • cell adhesion • cell differentiation • anchoring basal laminae to other ECM • blood clothing - clothing process, link to fibrin

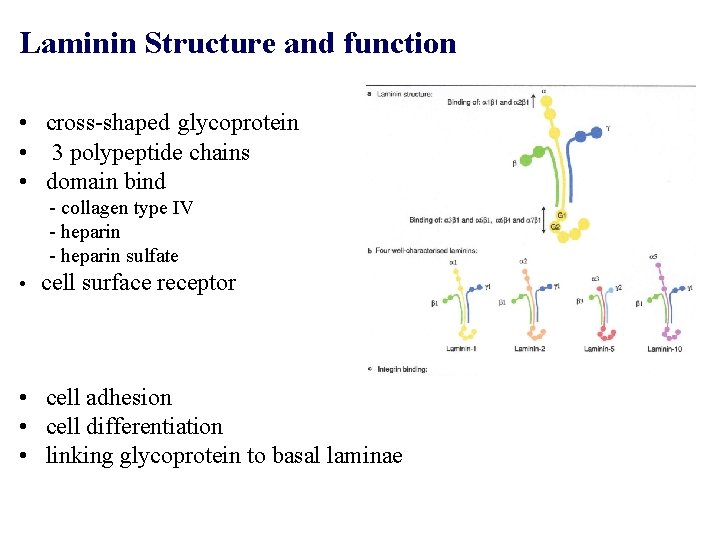
Laminin Structure and function • cross-shaped glycoprotein • 3 polypeptide chains • domain bind - collagen type IV - heparin sulfate • cell surface receptor • cell adhesion • cell differentiation • linking glycoprotein to basal laminae