Expression in mammalian cells Lab examples of cell
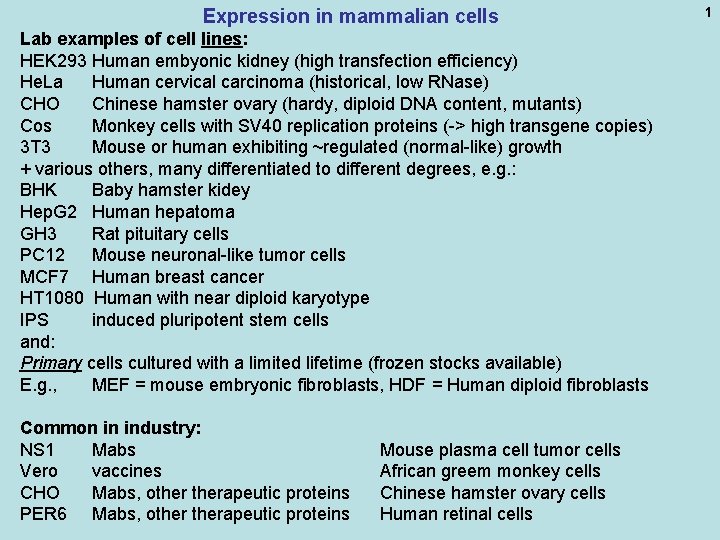
Expression in mammalian cells Lab examples of cell lines: HEK 293 Human embyonic kidney (high transfection efficiency) He. La Human cervical carcinoma (historical, low RNase) CHO Chinese hamster ovary (hardy, diploid DNA content, mutants) Cos Monkey cells with SV 40 replication proteins (-> high transgene copies) 3 T 3 Mouse or human exhibiting ~regulated (normal-like) growth + various others, many differentiated to different degrees, e. g. : BHK Baby hamster kidey Hep. G 2 Human hepatoma GH 3 Rat pituitary cells PC 12 Mouse neuronal-like tumor cells MCF 7 Human breast cancer HT 1080 Human with near diploid karyotype IPS induced pluripotent stem cells and: Primary cells cultured with a limited lifetime (frozen stocks available) E. g. , MEF = mouse embryonic fibroblasts, HDF = Human diploid fibroblasts Common in industry: NS 1 Mabs Vero vaccines CHO Mabs, otherapeutic proteins PER 6 Mabs, otherapeutic proteins Mouse plasma cell tumor cells African greem monkey cells Chinese hamster ovary cells Human retinal cells 1
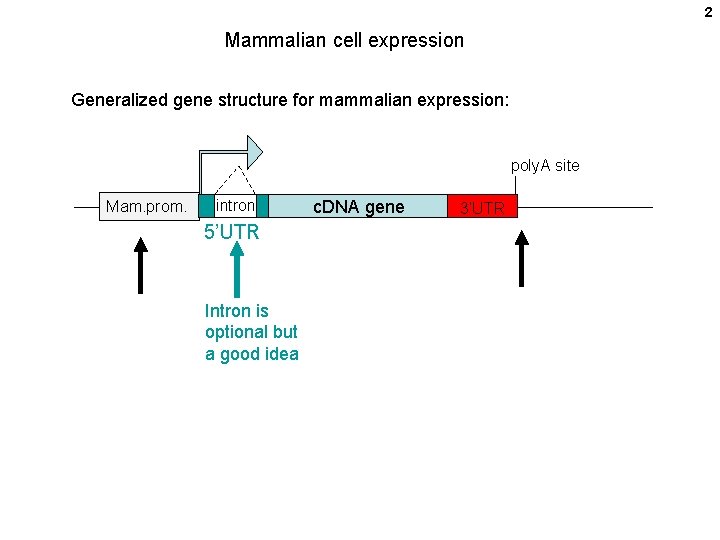
2 Mammalian cell expression Generalized gene structure for mammalian expression: poly. A site Mam. prom. intron 5’UTR Intron is optional but a good idea c. DNA gene 3’UTR

3 Popular mammalian cell promoters • • SV 40 Large. T Ag: Simian Virus 40 RSV LTR: Rous sarcoma virus MMTV: Mouse mammary tumor virus, glucocorticoid [Dex] inducible HSV TK: Herpes simplex virus, low expression Metallothionein: many sources, metal inducible, Cd++ CMV early: Cytomegalovirus, strong in most cell types Engineered inducible / repressible: tet-, ecdysone-, glucocorticoid- responsive (tet = tetracycline)
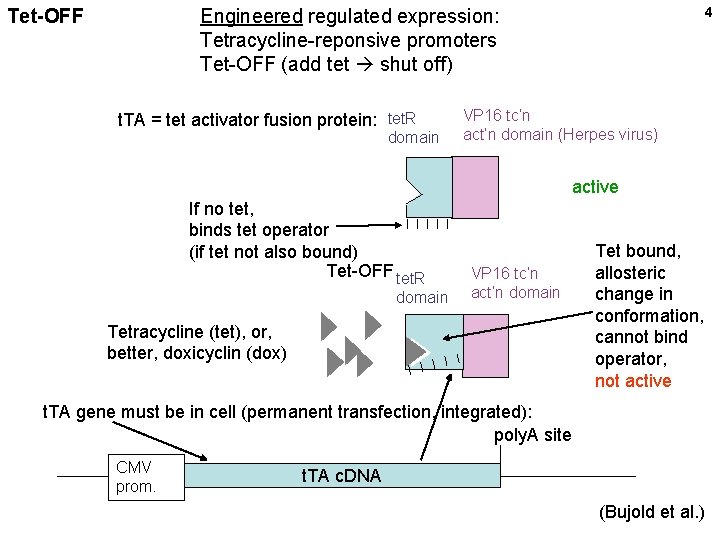
4 Engineered regulated expression: Tetracycline-reponsive promoters Tet-OFF (add tet shut off) Tet-OFF t. TA = tet activator fusion protein: tet. R domain VP 16 tc’n act’n domain (Herpes virus) active If no tet, binds tet operator (if tet not also bound) Tet-OFF tet. R domain VP 16 tc’n act’n domain Tetracycline (tet), or, better, doxicyclin (dox) Tet bound, allosteric change in conformation, cannot bind operator, not active t. TA gene must be in cell (permanent transfection, integrated): poly. A site CMV prom. t. TA c. DNA (Bujold et al. )
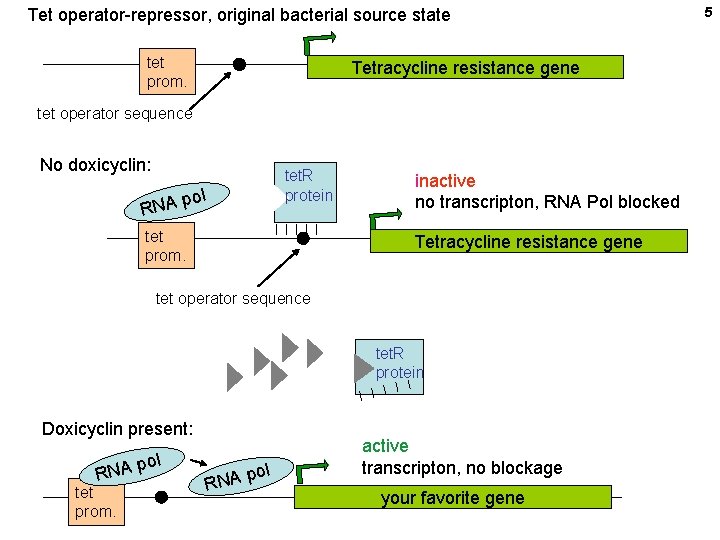
Tet operator-repressor, original bacterial source state tet prom. Tetracycline resistance gene tet operator sequence No doxicyclin: tet. R protein ol p RNA tet prom. inactive no transcripton, RNA Pol blocked Tetracycline resistance gene tet operator sequence tet. R protein Doxicyclin present: RN tet prom. A pol ol p RNA active transcripton, no blockage your favorite gene 5
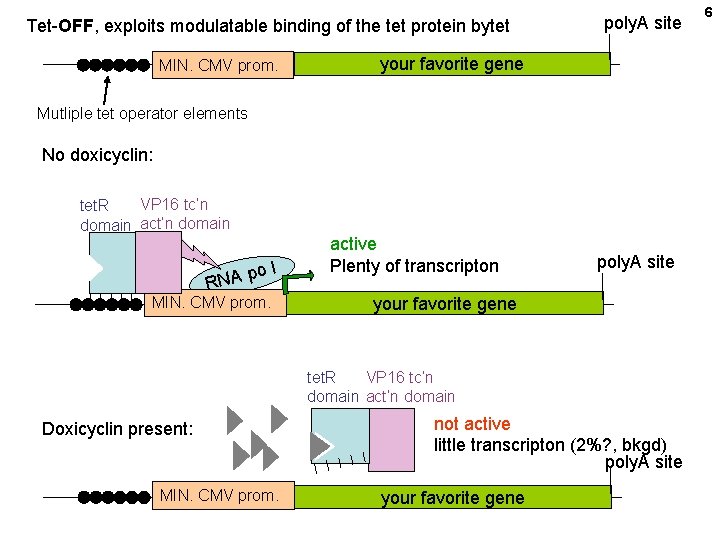
Tet-OFF, exploits modulatable binding of the tet protein bytet MIN. CMV prom. poly. A site your favorite gene Mutliple tet operator elements No doxicyclin: VP 16 tc’n tet. R domain act’n domain po RNA l MIN. CMV prom. active Plenty of transcripton poly. A site your favorite gene tet. R VP 16 tc’n domain act’n domain Doxicyclin present: MIN. CMV prom. not active little transcripton (2%? , bkgd) poly. A site your favorite gene 6
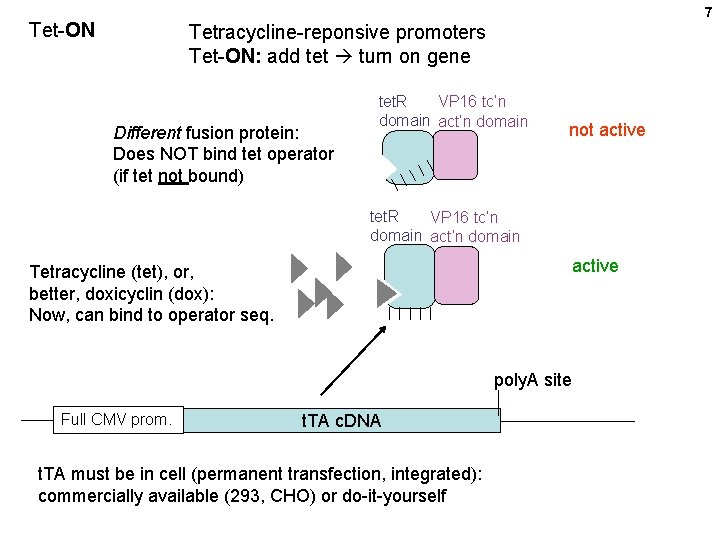
7 Tet-ON Tetracycline-reponsive promoters Tet-ON: add tet turn on gene Different fusion protein: Does NOT bind tet operator (if tet not bound) tet. R VP 16 tc’n domain act’n domain not active tet. R VP 16 tc’n domain active Tetracycline (tet), or, better, doxicyclin (dox): Now, can bind to operator seq. poly. A site Full CMV prom. t. TA c. DNA t. TA must be in cell (permanent transfection, integrated): commercially available (293, CHO) or do-it-yourself
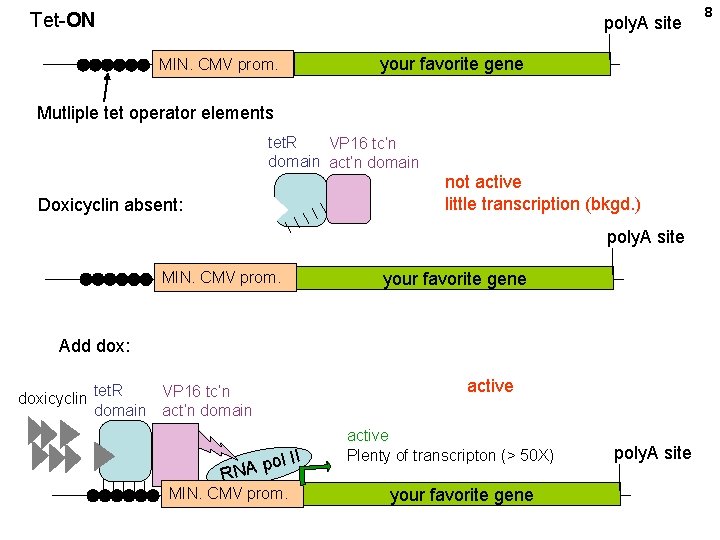
Tet-ON poly. A site MIN. CMV prom. your favorite gene Mutliple tet operator elements tet. R VP 16 tc’n domain act’n domain not active little transcription (bkgd. ) Doxicyclin absent: poly. A site MIN. CMV prom. your favorite gene Add dox: VP 16 tc’n doxicyclin tet. R domain act’n domain R ol II p A N MIN. CMV prom. active Plenty of transcripton (> 50 X) your favorite gene poly. A site 8
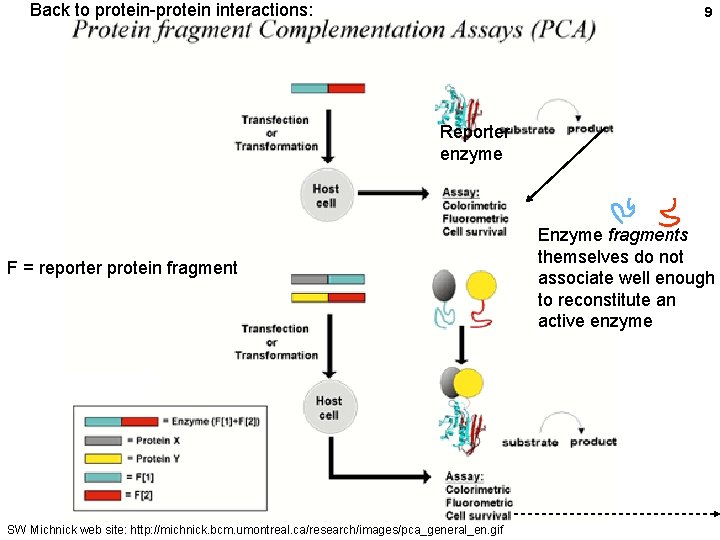
Back to protein-protein interactions: 9 Reporter enzyme F = reporter protein fragment SW Michnick web site: http: //michnick. bcm. umontreal. ca/research/images/pca_general_en. gif Enzyme fragments themselves do not associate well enough to reconstitute an active enzyme
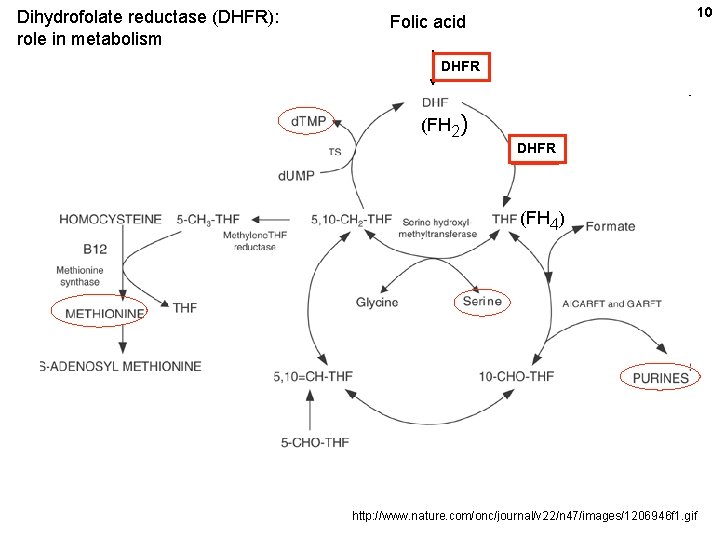
Dihydrofolate reductase (DHFR): role in metabolism 10 Folic acid DHFR (FH 2) DHFR (FH 4) http: //www. nature. com/onc/journal/v 22/n 47/images/1206946 f 1. gif
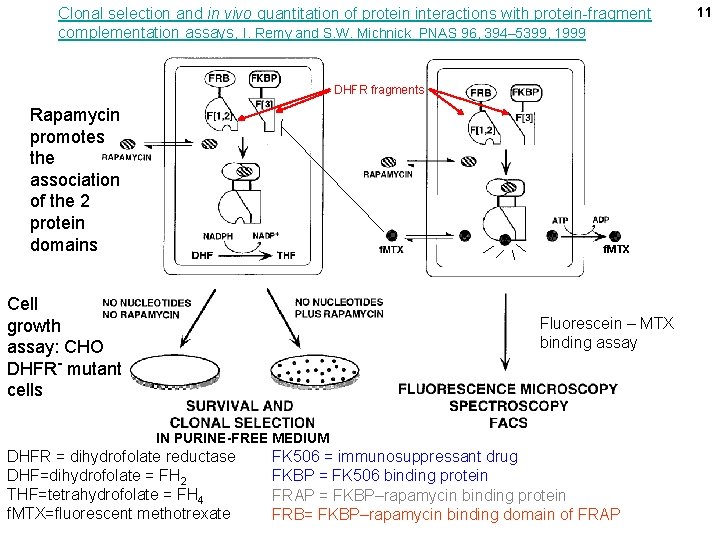
Clonal selection and in vivo quantitation of protein interactions with protein-fragment complementation assays, I. Remy and S. W. Michnick PNAS 96, 394– 5399, 1999 DHFR fragments Rapamycin promotes the association of the 2 protein domains f. MTX Cell growth assay: CHO DHFR- mutant cells Fluorescein – MTX binding assay IN PURINE-FREE MEDIUM DHFR = dihydrofolate reductase DHF=dihydrofolate = FH 2 THF=tetrahydrofolate = FH 4 f. MTX=fluorescent methotrexate FK 506 = immunosuppressant drug FKBP = FK 506 binding protein FRAP = FKBP–rapamycin binding protein FRB= FKBP–rapamycin binding domain of FRAP 11
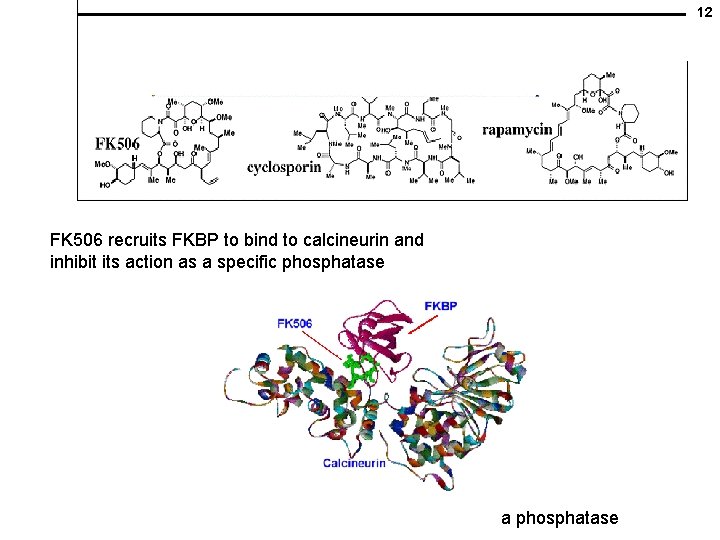
12 FK 506 recruits FKBP to bind to calcineurin and inhibit its action as a specific phosphatase a phosphatase
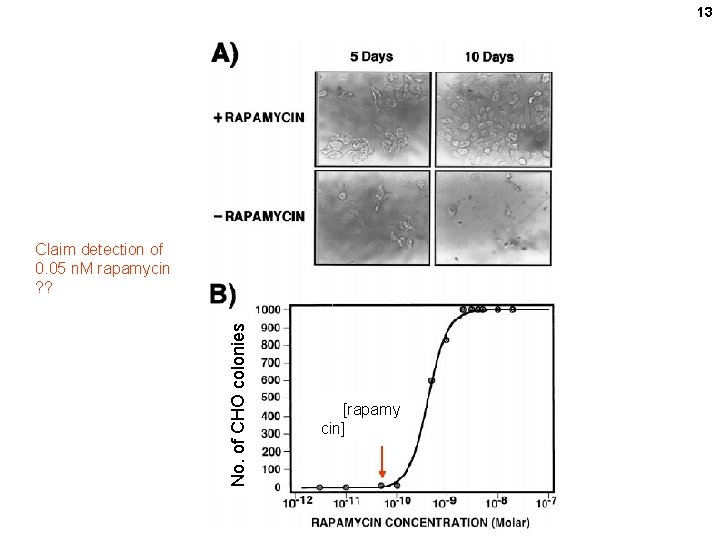
13 No. of CHO colonies Claim detection of 0. 05 n. M rapamycin ? ? [rapamy cin]
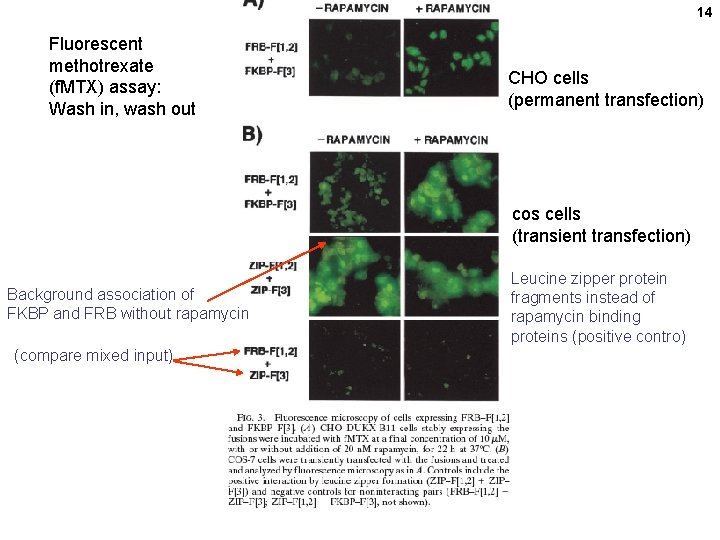
14 Fluorescent methotrexate (f. MTX) assay: Wash in, wash out CHO cells (permanent transfection) cos cells (transient transfection) Background association of FKBP and FRB without rapamycin (compare mixed input) Leucine zipper protein fragments instead of rapamycin binding proteins (positive contro)
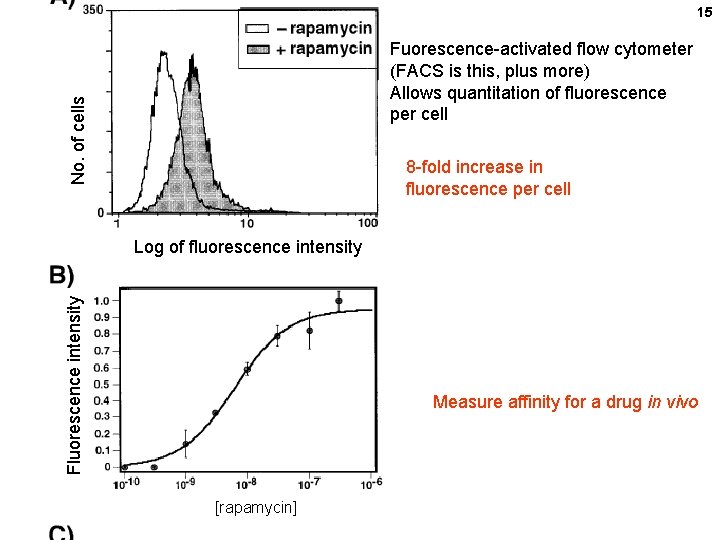
15 No. of cells Fuorescence-activated flow cytometer (FACS is this, plus more) Allows quantitation of fluorescence per cell 8 -fold increase in fluorescence per cell Fluorescence intensity Log of fluorescence intensity Measure affinity for a drug in vivo [rapamycin]
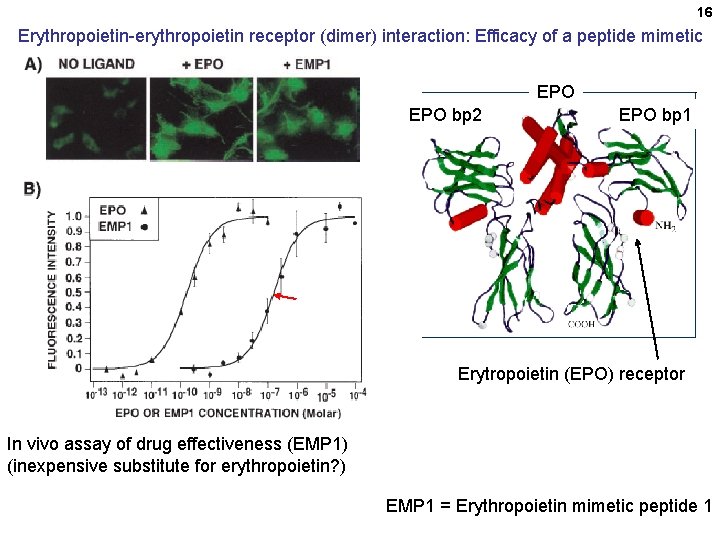
16 Erythropoietin-erythropoietin receptor (dimer) interaction: Efficacy of a peptide mimetic EPO bp 2 EPO bp 1 Erytropoietin (EPO) receptor In vivo assay of drug effectiveness (EMP 1) (inexpensive substitute for erythropoietin? ) EMP 1 = Erythropoietin mimetic peptide 1 Erythropoietin
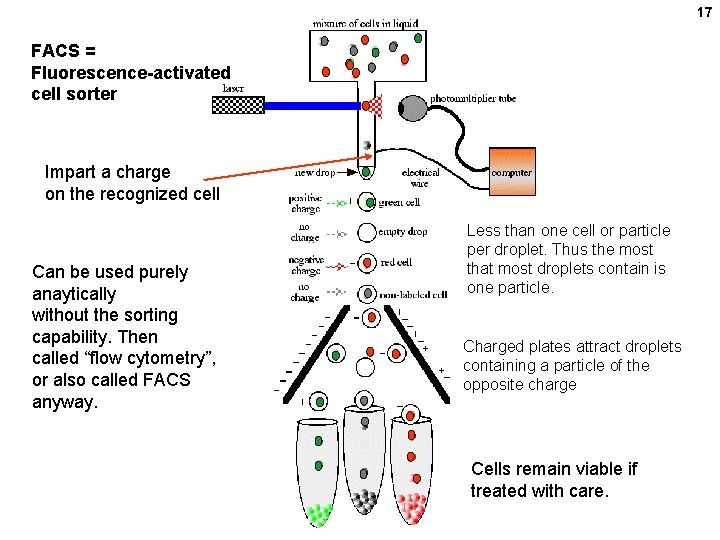
17 FACS = Fluorescence-activated cell sorter Impart a charge on the recognized cell Can be used purely anaytically without the sorting capability. Then called “flow cytometry”, or also called FACS anyway. Less than one cell or particle per droplet. Thus the most that most droplets contain is one particle. Charged plates attract droplets containing a particle of the opposite charge Cells remain viable if treated with care.
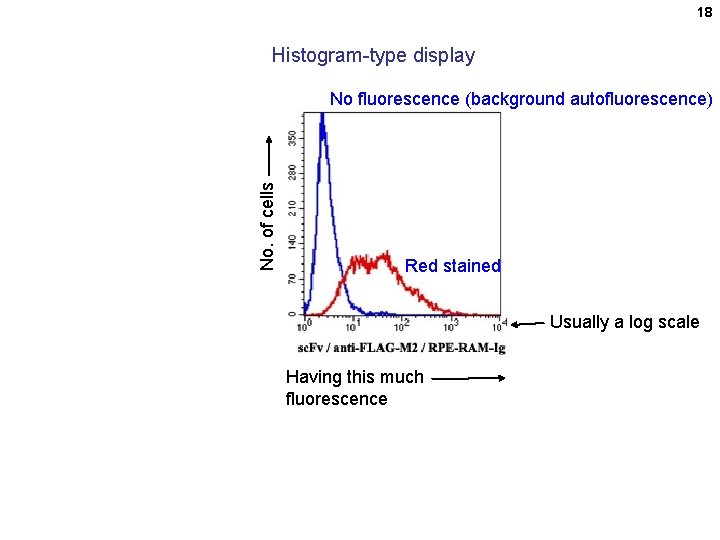
18 Histogram-type display No. of cells No fluorescence (background autofluorescence) Red stained Usually a log scale Having this much fluorescence
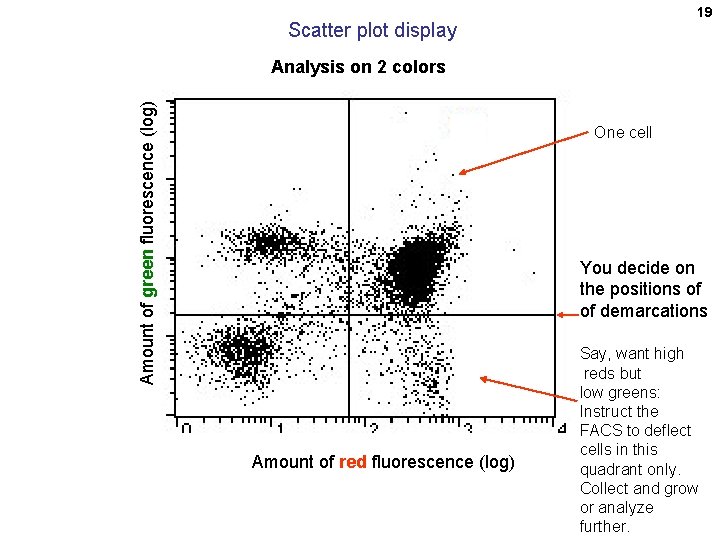
19 Scatter plot display Amount of green fluorescence (log) Analysis on 2 colors One cell You decide on the positions of of demarcations Amount of red fluorescence (log) Say, want high reds but low greens: Instruct the FACS to deflect cells in this quadrant only. Collect and grow or analyze further.
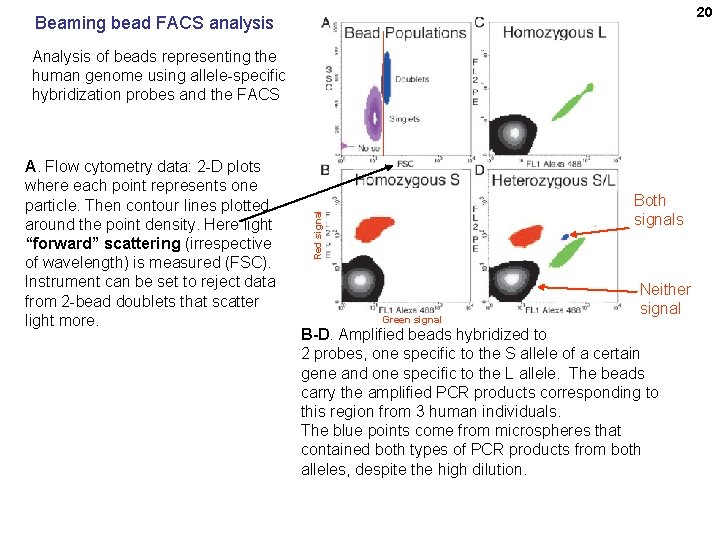
20 Beaming bead FACS analysis Analysis of beads representing the human genome using allele-specific hybridization probes and the FACS Both signals Red signal A. Flow cytometry data: 2 -D plots where each point represents one particle. Then contour lines plotted around the point density. Here light “forward” scattering (irrespective of wavelength) is measured (FSC). Instrument can be set to reject data from 2 -bead doublets that scatter light more. Green signal Neither signal B-D. Amplified beads hybridized to 2 probes, one specific to the S allele of a certain gene and one specific to the L allele. The beads carry the amplified PCR products corresponding to this region from 3 human individuals. The blue points come from microspheres that contained both types of PCR products from both alleles, despite the high dilution.

21 Biotechnology methods to study transcriptional regulation in cells Mainly, use of reporter proteins whose c. DNA sequence is linked to the promoter. First, a synopsis of promoter structure:
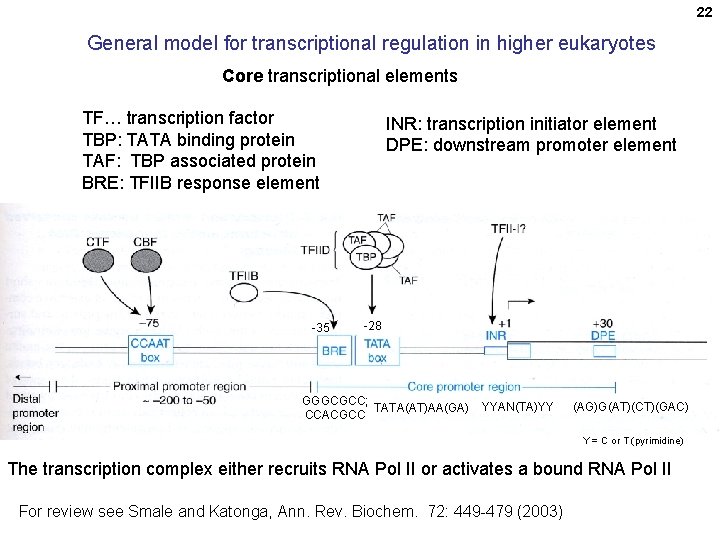
22 General model for transcriptional regulation in higher eukaryotes Core transcriptional elements TF… transcription factor TBP: TATA binding protein TAF: TBP associated protein BRE: TFIIB response element -35 INR: transcription initiator element DPE: downstream promoter element -28 GGGCGCC; TATA(AT)AA(GA) CCACGCC YYAN(TA)YY (AG)G(AT)(CT)(GAC) Y = C or T (pyrimidine) The transcription complex either recruits RNA Pol II or activates a bound RNA Pol II For review see Smale and Katonga, Ann. Rev. Biochem. 72: 449 -479 (2003)
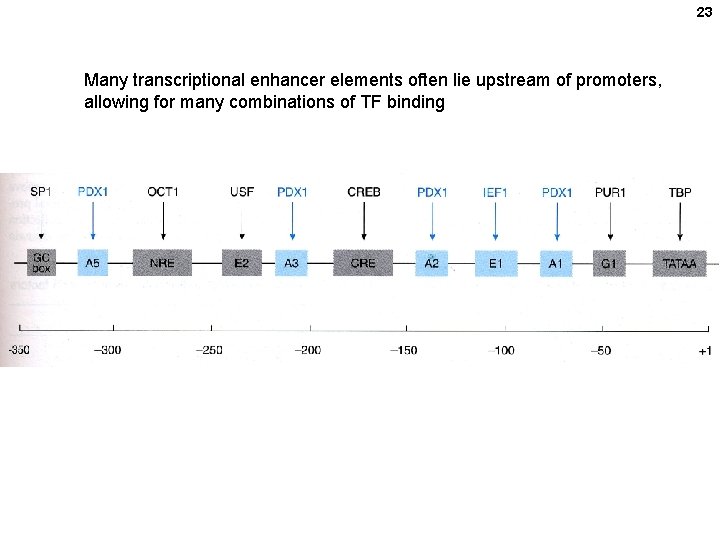
23 Many transcriptional enhancer elements often lie upstream of promoters, allowing for many combinations of TF binding

24 Got this far
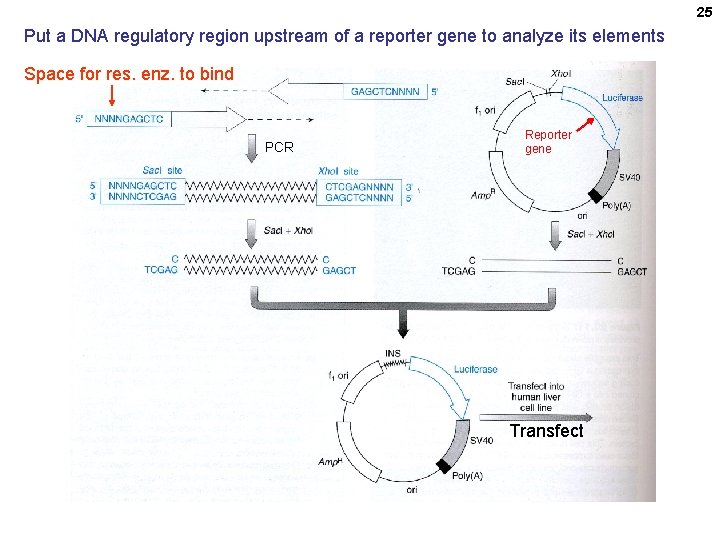
25 Put a DNA regulatory region upstream of a reporter gene to analyze its elements Space for res. enz. to bind PCR Reporter gene Transfect
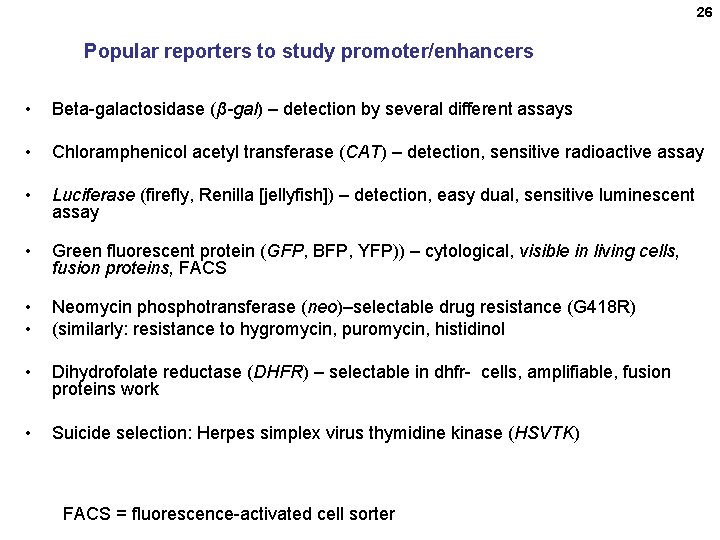
26 Popular reporters to study promoter/enhancers • Beta-galactosidase (β-gal) – detection by several different assays • Chloramphenicol acetyl transferase (CAT) – detection, sensitive radioactive assay • Luciferase (firefly, Renilla [jellyfish]) – detection, easy dual, sensitive luminescent assay • Green fluorescent protein (GFP, BFP, YFP)) – cytological, visible in living cells, fusion proteins, FACS • • Neomycin phosphotransferase (neo)–selectable drug resistance (G 418 R) (similarly: resistance to hygromycin, puromycin, histidinol • Dihydrofolate reductase (DHFR) – selectable in dhfr- cells, amplifiable, fusion proteins work • Suicide selection: Herpes simplex virus thymidine kinase (HSVTK) FACS = fluorescence-activated cell sorter
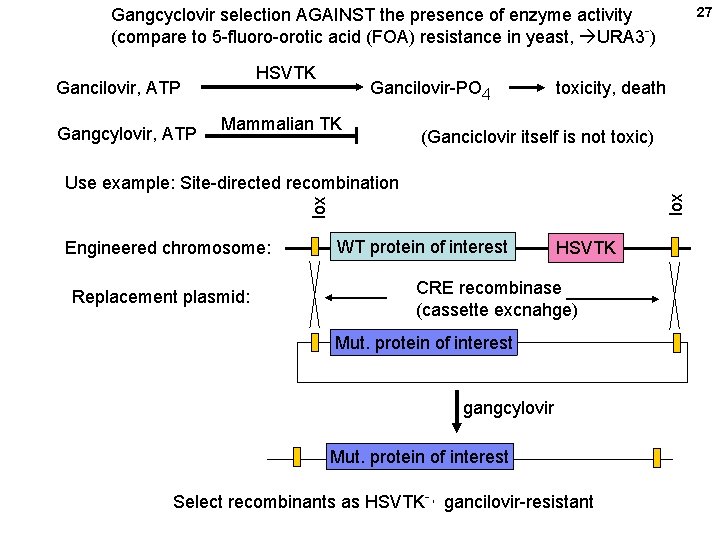
27 Gangcyclovir selection AGAINST the presence of enzyme activity (compare to 5 -fluoro-orotic acid (FOA) resistance in yeast, URA 3 -) HSVTK Gancilovir, ATP Gangcylovir, ATP Gancilovir-PO 4 Mammalian TK toxicity, death (Ganciclovir itself is not toxic) lox Use example: Site-directed recombination Engineered chromosome: Replacement plasmid: WT protein of interest HSVTK CRE recombinase (cassette excnahge) Mut. protein of interest gangcylovir Mut. protein of interest Select recombinants as HSVTK-, gancilovir-resistant
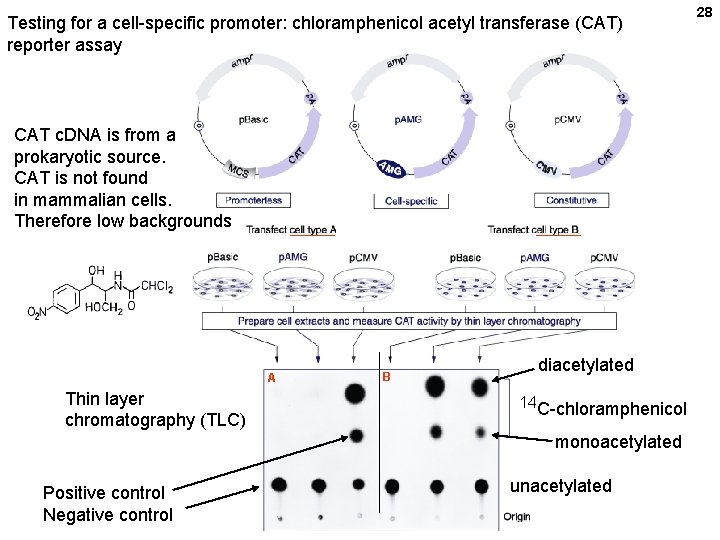
Testing for a cell-specific promoter: chloramphenicol acetyl transferase (CAT) reporter assay CAT c. DNA is from a prokaryotic source. CAT is not found in mammalian cells. Therefore low backgrounds A Thin layer chromatography (TLC) B diacetylated 14 C-chloramphenicol monoacetylated Positive control Negative control unacetylated 28

29 Reporter enzyme substrates for different purposes Substrates for beta-galactosidase, for example: • ONPG (ortho-nitrophenyl-beta-galactoside) – spectrophotometric measurement (420 nm – blue color – simplest) • X-gal (5 -Bromo-4 -chloro-3 -indolyl-ß-D-galactoside) – blue precipitate - for cytology or colony detection • Umbelliferyl–galactoside (-> umbelliferone, fluorescent, reading in a fluorimeter allows more sensitive quantification than spectrophotometry) • Galacton-STAR or some such (-> chemiluminescent product = emission of light, so lower background than fluorescence) • Lactose (glucose-beta-galactose disaccharide) – allows growth if hydrolyzed; growth phenotype. For microbial cells usually.
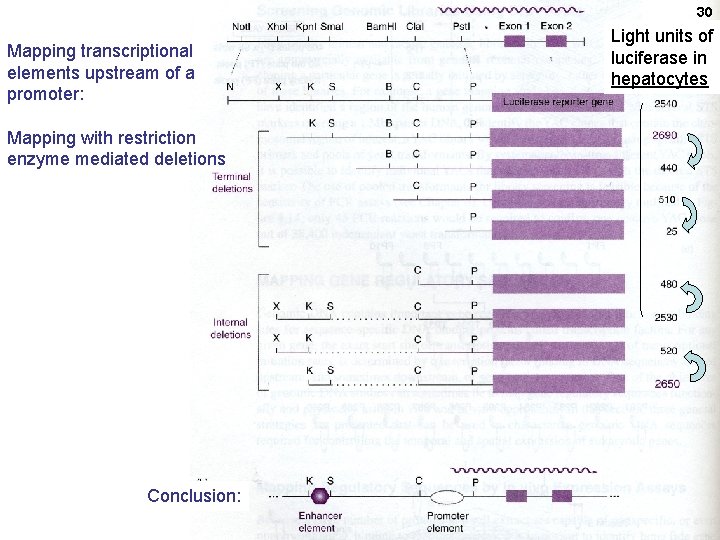
30 Mapping transcriptional elements upstream of a promoter: Mapping with restriction enzyme mediated deletions Conclusion: Light units of luciferase in hepatocytes
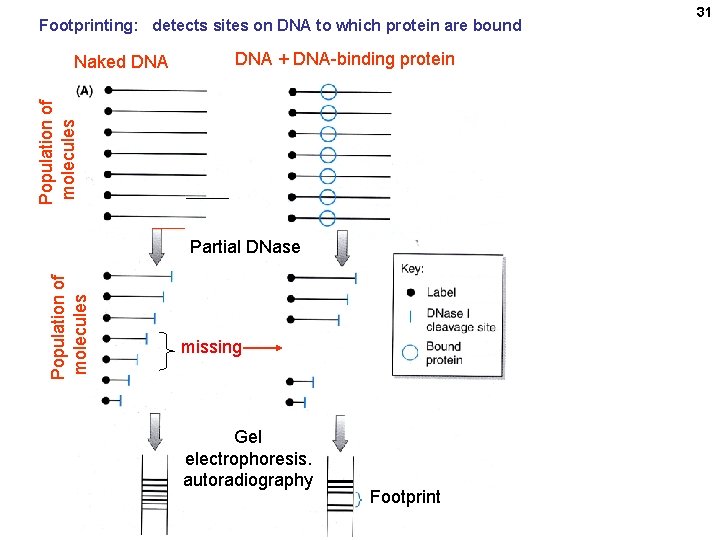
Footprinting: detects sites on DNA to which protein are bound DNA + DNA-binding protein Population of molecules Naked DNA Population of molecules Partial DNase missing Gel electrophoresis. autoradiography Footprint 31
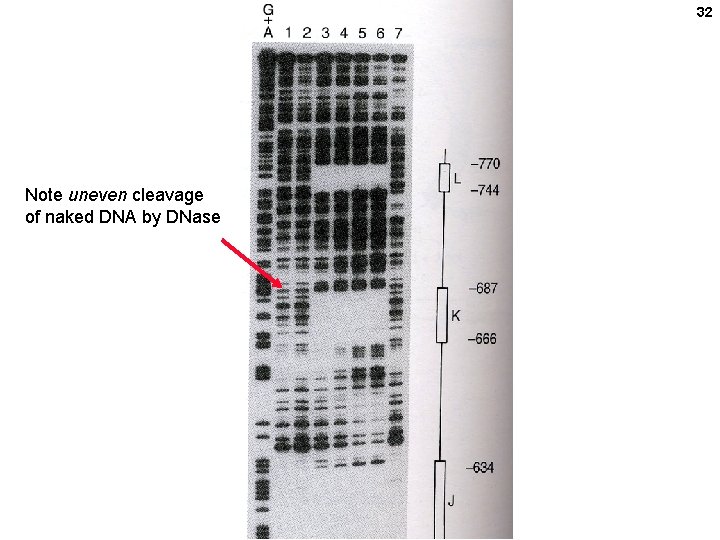
32 Note uneven cleavage of naked DNA by DNase
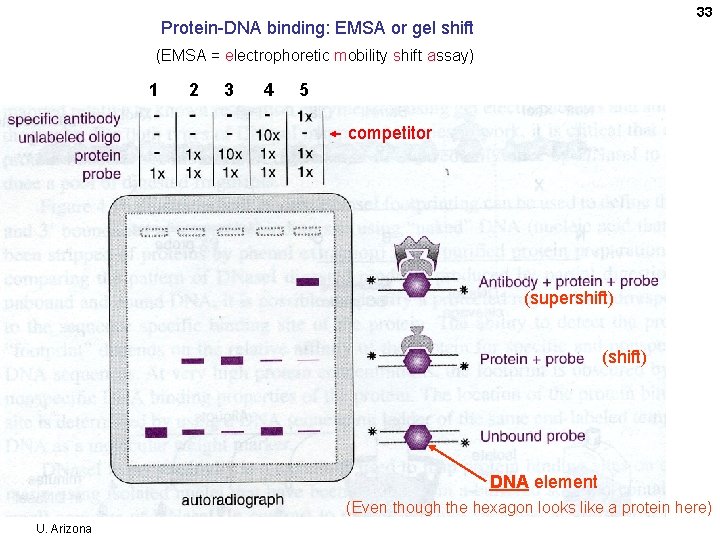
33 Protein-DNA binding: EMSA or gel shift (EMSA = electrophoretic mobility shift assay) 1 2 3 4 5 competitor (supershift) (shift) DNA element (Even though the hexagon looks like a protein here) U. Arizona
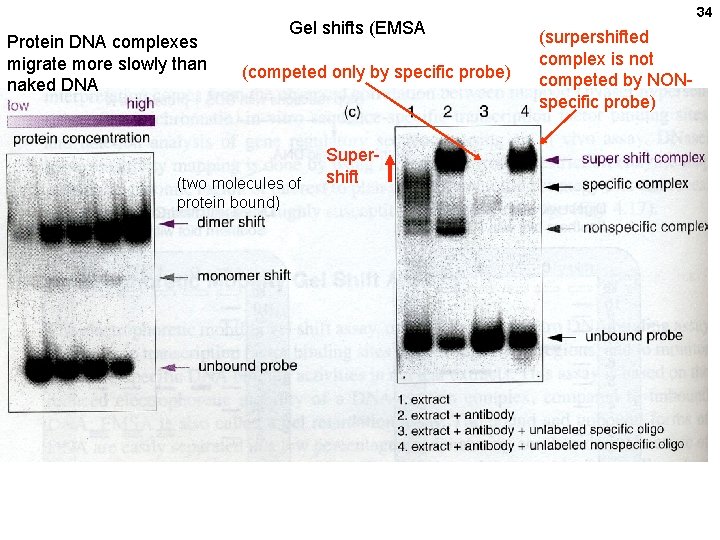
Protein DNA complexes migrate more slowly than naked DNA Gel shifts (EMSA (competed only by specific probe) (two molecules of protein bound) Supershift 34 (surpershifted complex is not competed by NONspecific probe)
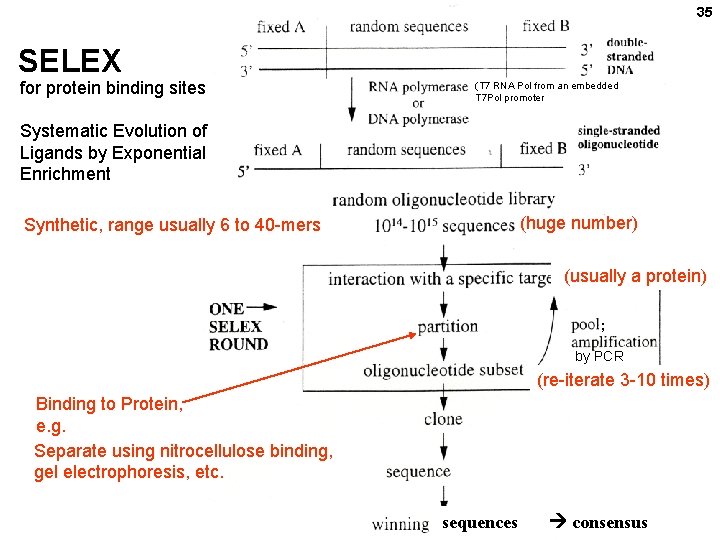
35 SELEX for protein binding sites (T 7 RNA Pol from an embedded T 7 Pol promoter Systematic Evolution of Ligands by Exponential Enrichment (huge number) Synthetic, range usually 6 to 40 -mers (usually a protein) ; by PCR (re-iterate 3 -10 times) Binding to Protein, e. g. Separate using nitrocellulose binding, gel electrophoresis, etc. sequences consensus
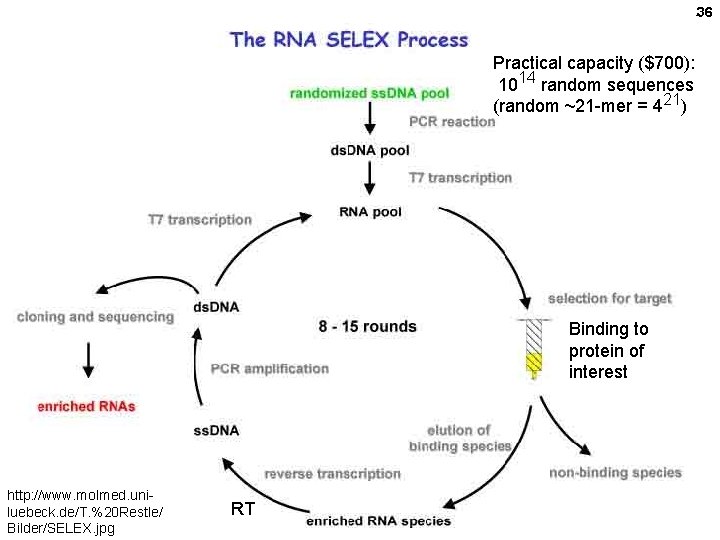
36 Practical capacity ($700): 1014 random sequences (random ~21 -mer = 421) Binding to protein of interest http: //www. molmed. uniluebeck. de/T. %20 Restle/ Bilder/SELEX. jpg RT

37 Binding site for a “puf “ protein, implicated in m. RNA degradation PUM 2, a novel murine puf protein, and its consensus RNA-binding site . White EK, Moore-Jarrett T, Ruley HE. RNA. 2001 Dec; 7(12): 1855 -66 20 -mer Consensus: Description Nucleic acid degenerate base abbreviations Co de Integ er Base Name Meani ng Compleme nt A 1 Adenine A T C 2 Cytosine C G G 3 Guanine G C T 4 Thymine T A U 4 Uracil U A R 5 (Pu. Rine) G|A Y Y 6 (PYrimidine) T|C R K 7 (Keto) G|T M M 8 (AMino) A|C K S 9 Strong interaction (3 H bonds) G|C S W 10 Weak interaction (2 H bonds) A|T W B 11 Not-A (B follows A) G|T|C V D 12 Not-C (D follows C) G|A|T H H 13 Not-G (H follows G) A|T|C D V 14 Not-T (or U) (V follows U) G|A|C B N, X 15 ANy nucleotide G|A|T| C N - 16 Gap of indeterminate length Gap -
- Slides: 37